Comparative ultrastructure of cells and cuticle in the anterior chamber and papillate region of Porcellioscaber (Crustacea, Isopoda) hindgut
- PMID: 30564048
- PMCID: PMC6288245
- DOI: 10.3897/zookeys.801.22395
Comparative ultrastructure of cells and cuticle in the anterior chamber and papillate region of Porcellioscaber (Crustacea, Isopoda) hindgut
Abstract
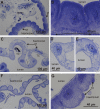
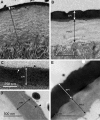
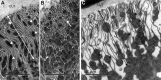
Isopod hindgut consists of two anatomical and functional parts, the anterior chamber, and the papillate region. This study provides a detailed ultrastructural comparison of epithelial cells in the anterior chamber and the papillate region with focus on cuticle ultrastructure, apical and basal plasma membrane labyrinths, and cell junctions. Na+/K+-ATPase activity in the hindgut epithelial cells was demonstrated by cytochemical localisation. The main difference in cuticle ultrastructure is in the thickness of epicuticle which is almost as thick as the procuticle in the papillate region and only about one sixth of the thickness of procuticle in the anterior chamber. The apical plasma membrane in both hindgut regions forms an apical plasma membrane labyrinth of cytoplasmic strands and extracellular spaces. In the papillate region the membranous infoldings are deeper and the extracellular spaces are wider. The basal plasma membrane is extensively infolded and associated with numerous mitochondria in the papillate region, while it forms relatively scarce basal infoldings in the anterior chamber. The junctional complex in both hindgut regions consists of adherens and septate junctions. Septate junctions are more extensive in the papillate region. Na+/K+-ATPase was located mostly in the apical plasma membranes in both hindgut regions. The ultrastructural features of hindgut cuticle are discussed in comparison to exoskeletal cuticle and to cuticles of other arthropod transporting epithelia from the perspective of their mechanical properties and permeability. The morphology of apical and basal plasma membranes and localisation of Na+/K+-ATPase are compared with other arthropod-transporting epithelia according to different functions of the anterior chamber and the papillate region.
Keywords: cell junctions; digestive system; extracellular matrix; ion transporting epithelium; plasma membrane labyrinth.
Figures






References
-
- Berridge MJ, Gupta BL. (1967) Fine-structural changes in relation to ion and water transport in the rectal papillae of the blowfly, Calliphora. Journal of Cell Science 2: 89–112. http://jcs.biologists.org/content/2/1/89 - PubMed
-
- Bettica A, Witkus R, Vernon GM. (1987) Ultrastructure of the foregut-hindgut junction in Porcellioscaber Latreille. Journal of Crustacean Biology 7(4): 619–623. https://www.jstor.org/stable/1548647
-
- Boudour-Boucheker N, Boulo V, Charmantier-Daures M, Grousset E, Anger K, Charmantier G, Lorin-Nebel C. (2014) Differential distribution of V-type H+-ATPase and Na+/K+-ATPase in the branchial chamber of the palaemonid shrimp Macrobrachiumamazonicum. Cell and Tissue Research 357(1): 195–206. 10.1007/s00441-014-1845-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
