Bacteria-Host Interactions in Multiple Sclerosis
- PMID: 30564215
- PMCID: PMC6288311
- DOI: 10.3389/fmicb.2018.02966
Bacteria-Host Interactions in Multiple Sclerosis
Abstract
Multiple sclerosis (MS) is caused by a complex interaction of genetic and environmental factors. Numerous causative factors have been identified that play a role in MS, including exposure to bacteria. Mycobacteria, Chlamydia pneumoniae, Helicobacter pylori, and other bacteria have been proposed as risk factors for MS with different mechanisms of action. Conversely, some pathogens may have a protective effect on its etiology. In terms of acquired immunity, molecular mimicry has been hypothesized as the mechanism by which bacterial structures such as DNA, the cell wall, and intracytoplasmic components can activate autoreactive T cells or produce autoantibodies in certain host genetic backgrounds of susceptible individuals. In innate immunity, Toll-like receptors play an essential role in combating invading bacteria, and their activation leads to the release of cytokines or chemokines that mediate effective adaptive immune responses. These receptors may also be involved in central nervous system autoimmunity, and their contribution depends on the infection site and on the pathogen. We have reviewed the current knowledge of the influence of bacteria on MS development, emphasizing the potential mechanisms of action by which bacteria affect MS initiation and/or progression.
Keywords: acquired immunity; bacteria; innate immunity; multiple sclerosis; pathogen–host interaction.
Figures
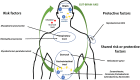
 through circumventricular organs characterized into sensory and secretory organs, comprising the subfornical organ, vascular organ of the lamina terminalis, area postrema, median eminence, neurohypophysis, sub-commissural organ, choroid plexus, and pineal gland (Weiss and Schaible, 2015);
through circumventricular organs characterized into sensory and secretory organs, comprising the subfornical organ, vascular organ of the lamina terminalis, area postrema, median eminence, neurohypophysis, sub-commissural organ, choroid plexus, and pineal gland (Weiss and Schaible, 2015);  through the BBB or the blood–cerebrospinal fluid barrier (Ganong, 2000);
through the BBB or the blood–cerebrospinal fluid barrier (Ganong, 2000);  through the GALT, which consists of Peyer’s patches, intraepithelial lymphocytes, and lamina propria lymphocytes (Doran et al., 2013);
through the GALT, which consists of Peyer’s patches, intraepithelial lymphocytes, and lamina propria lymphocytes (Doran et al., 2013);  through gut microbiota by a bi-directional communication system (gut–brain axis), including the autonomic nervous system, the enteric nervous system, the vagus nerve, and the hypothalamic pituitary adrenal axis (Wucherpfennig, 2001); and
through gut microbiota by a bi-directional communication system (gut–brain axis), including the autonomic nervous system, the enteric nervous system, the vagus nerve, and the hypothalamic pituitary adrenal axis (Wucherpfennig, 2001); and  through meningeal lymphatics capable of draining CNS macromolecules into the cervical lymph nodes. Inflammation is known to induce expansion of the local lymphatic vasculature in peripheral tissues and, hence, it is likely that bacterial exposure autoimmune cell activation will occur (Cosorich et al., 2017).
through meningeal lymphatics capable of draining CNS macromolecules into the cervical lymph nodes. Inflammation is known to induce expansion of the local lymphatic vasculature in peripheral tissues and, hence, it is likely that bacterial exposure autoimmune cell activation will occur (Cosorich et al., 2017).References
-
- Arsenault R. J., Li Y., Maattanen P., Scruten E., Doig K., Potter A., et al. (2013). Altered Toll-like receptor 9 signaling in Mycobacterium avium subsp. paratuberculosis-infected bovine monocytes reveals potential therapeutic targets. Infect. Immun. 81 226–237. 10.1128/IAI.00785-12 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

