Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning
- PMID: 30564236
- PMCID: PMC6288292
- DOI: 10.3389/fimmu.2018.02837
Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning
Abstract
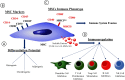
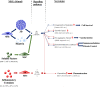
Mesenchymal stromal cells (MSCs) are self-renewing, culture-expandable adult stem cells that have been isolated from a variety of tissues, and possess multipotent differentiation capacity, immunomodulatory properties, and are relatively non-immunogenic. Due to this unique set of characteristics, these cells have attracted great interest in the field of regenerative medicine and have been shown to possess pronounced therapeutic potential in many different pathologies. MSCs' mode of action involves a strong paracrine component resulting from the high levels of bioactive molecules they secrete in response to the local microenvironment. For this reason, MSCs' secretome is currently being explored in several clinical contexts, either using MSC-conditioned media (CM) or purified MSC-derived extracellular vesicles (EVs) to modulate tissue response to a wide array of injuries. Rather than being a constant mixture of molecular factors, MSCs' secretome is known to be dependent on the diverse stimuli present in the microenvironment that MSCs encounter. As such, the composition of the MSCs' secretome can be modulated by preconditioning the MSCs during in vitro culture. This manuscript reviews the existent literature on how preconditioning of MSCs affects the therapeutic potential of their secretome, focusing on MSCs' immunomodulatory and regenerative features, thereby providing new insights for the therapeutic use of MSCs' secretome.
Keywords: MSCs (Mesenchymal Stromal Cells); immunomodulation; pre-conditioning; regeneration; secretome; therapeutic potential.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

