Galectin-Glycan Interactions as Regulators of B Cell Immunity
- PMID: 30564237
- PMCID: PMC6288978
- DOI: 10.3389/fimmu.2018.02839
Galectin-Glycan Interactions as Regulators of B Cell Immunity
Abstract
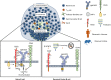
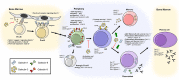
Cell surface glycans and their glycan-binding partners (lectins) have generally been recognized as adhesive assemblies with neighbor cells or matrix scaffolds in organs and the blood stream. However, our understanding of the roles for glycan-lectin interactions in immunity has expanded substantially to include regulation of nearly every stage of an immune response, from pathogen sensing to immune contraction. In this Mini-Review, we discuss the role of the ß-galactoside-binding lectins known as galectins specifically in the regulation of B-lymphocyte (B cell) development, activation, and differentiation. In particular, we highlight several recent studies revealing new roles for galectin (Gal)-9 in the modulation of B cell receptor-mediated signaling and activation in mouse and man. The roles for cell surface glycosylation, especially I-branching of N-glycans synthesized by the glycosyltransferase GCNT2, in the regulation of Gal-9 binding activity are also detailed. Finally, we consider how dysregulation of these factors may contribute to aberrant immune activation and autoimmune disease.
Keywords: B cell activation; B cell receptor; B cells; GCNT2; I-antigen; I-branch; galectin.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

