TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells
- PMID: 30564247
- PMCID: PMC6288997
- DOI: 10.3389/fimmu.2018.02864
TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells
Abstract
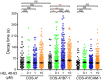
T cell activation is initiated upon ligand engagement of the T cell receptor (TCR) and costimulatory receptors. The CD28 molecule acts as a major costimulatory receptor in promoting full activation of naive T cells. However, despite extensive studies, why naive T cell activation requires concurrent stimulation of both the TCR and costimulatory receptors remains poorly understood. Here, we explore this issue by analyzing calcium response as a key early signaling event to elicit T cell activation. Experiments using mouse naive CD4+ T cells showed that engagement of the TCR or CD28 with the respective cognate ligand was able to trigger a rise in fluctuating calcium mobilization levels, as shown by the frequency and average response magnitude of the reacting cells compared with basal levels occurred in unstimulated cells. The engagement of both TCR and CD28 enabled a further increase of these two metrics. However, such increases did not sufficiently explain the importance of the CD28 pathways to the functionally relevant calcium responses in T cell activation. Through the autocorrelation analysis of calcium time series data, we found that combined but not separate TCR and CD28 stimulation significantly prolonged the average decay time (τ) of the calcium signal amplitudes determined with the autocorrelation function, compared with its value in unstimulated cells. This increasement of decay time (τ) uniquely characterizes the fluctuating calcium response triggered by concurrent stimulation of TCR and CD28, as it could not be achieved with either stronger TCR stimuli or by co-engaging both TCR and LFA-1, and likely represents an important feature of competent early signaling to provoke efficient T cell activation. Our work has thus provided new insights into the interplay between the TCR and CD28 early signaling pathways critical to trigger naive T cell activation.
Keywords: CD28; T cell activation; TCR–T cell receptor; calcium signaling; co-stimulation; naive T cells.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

