The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function
- PMID: 30564248
- PMCID: PMC6288403
- DOI: 10.3389/fimmu.2018.02867
The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function
Abstract
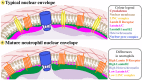
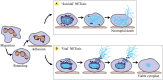
Neutrophil nuclear morphology has historically been used in haematology for neutrophil identification and characterisation, but its exact role in neutrophil function has remained enigmatic. During maturation, segmentation of the neutrophil nucleus into its mature, multi-lobulated shape is accompanied by distinct changes in nuclear envelope composition, resulting in a unique nucleus that is believed to be imbued with extraordinary nuclear flexibility. As a rate-limiting factor for cell migration, nuclear morphology and biomechanics are particularly important in the context of neutrophil migration during immune responses. Being an extremely plastic and fast migrating cell type, it is to be expected that neutrophils have an especially deformable nucleus. However, many questions still surround the dynamic capacities of the neutrophil nucleus, and which nuclear and cytoskeletal elements determine these dynamics. The biomechanics of the neutrophil nucleus should also be considered for their influences on the production of neutrophil extracellular traps (NETs), given this process sees the release of chromatin "nets" from nucleoplasm to extracellular space. Although past studies have investigated neutrophil nuclear composition and shape, in a new era of more sophisticated biomechanical and genetic techniques, 3D migration studies, and higher resolution microscopy we now have the ability to further investigate and understand neutrophil nuclear plasticity at an unprecedented level. This review addresses what is currently understood about neutrophil nuclear structure and its role in migration and the release of NETs, whilst highlighting open questions surrounding neutrophil nuclear dynamics.
Keywords: NETs; lamin B receptor; lamins; leukocytes; migration; neutrophil extracellular traps; neutrophils; nucleus.
Figures


References
-
- Hutchison CJ, Bridger JM, Cox LS, Kill IR. Weaving a pattern from disparate threads: Lamin function in nuclear assembly and DNA replication. J Cell Sci. (1994) 107:3259–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

