Worldwide practices on flexible endoscope reprocessing
- PMID: 30564309
- PMCID: PMC6296091
- DOI: 10.1186/s13756-018-0446-6
Worldwide practices on flexible endoscope reprocessing
Abstract
Background: Endoscopy related infections represent an important threat for healthcare systems worldwide. Recent outbreaks of infections with multidrug resistant micro-organisms have highlighted the problems of contaminated endoscopes. Endoscopes at highest risk for contamination have intricate mechanisms, multiple internal channels and narrow lumens that are especially problematic to clean. In light of raised awareness about the necessity for meticulous reprocessing of all types of endoscopes, a call for international collaboration is needed. An overview is presented on current practices for endoscope reprocessing in facilities worldwide.
Method: An electronic survey was developed and disseminated by the International Society for Antimicrobials and Chemotherapy. The survey consisted of 50 questions aimed at assessing the reprocessing of flexible endoscopes internationally. It covered three core elements: stakeholder involvement, assessment of perceived risks, and reprocessing process.
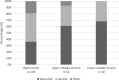
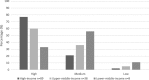
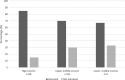
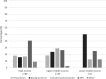
Results: The survey received a total of 165 completed responses from 39 countries. It is evident that most facilities, 82% (n = 136), have a standard operating procedure. There is, however a lot of variation within the flexible endoscope reprocessing practices observed. The need for regular training and education of reprocessing practitioners were identified by 50% (n = 83) of the respondents as main concerns that need to be addressed in order to increase patient safety in endoscope reprocessing procedures.
Conclusion: This international survey on current flexible endoscope reprocessing identified a large variation for reprocessing practices among different health care facilities/countries. A standardised education and training programme with a competency assessment is essential to prevent reprocessing lapses and improve patient safety.
Keywords: AER; Flexible endoscopes; Guidelines; Monitoring; Reprocessing.
Conflict of interest statement
The authors declare that they have no competing interests.Not applicable.Not applicable.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Rutala WA, Weber DJ, Committee HICPA Guidedline for disinfection and sterilization in healthcare facilities. CDC, Atlanta, GA 2008.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

