Urothelial Senescence in the Pathophysiology of Diabetic Bladder Dysfunction-A Novel Hypothesis
- PMID: 30564582
- PMCID: PMC6288180
- DOI: 10.3389/fsurg.2018.00072
Urothelial Senescence in the Pathophysiology of Diabetic Bladder Dysfunction-A Novel Hypothesis
Abstract
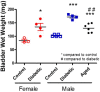
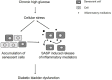
Diabetic bladder dysfunction (DBD) is a well-recognized and common symptom affecting up to 50% of all diabetic patients. DBD has a broad range of clinical presentations ranging from overactive to underactive bladder symptoms that develops in middle-aged to elderly patients with long standing and poorly controlled diabetes. Low efficacy of current therapeutics and lifestyle interventions combined with high national healthcare costs highlight the need for more research into bladder dysfunction pathophysiology and novel treatment options. Cellular senescence is an age-related physiologic process in which cells undergo irreversible growth arrest induced by replicative exhaustion and damaging insults. While controlled senescence negatively regulates cell proliferation and promotes tissue regeneration, uncontrolled senescence is known to result in tissue dysfunction through enhanced secretion of inflammatory factors. This review presents previous scientific findings and current hypotheses that characterize diabetic bladder dysfunction. Further, we propose the novel hypothesis that cellular senescence within the urothelial layer of the bladder contributes to the pro-inflammatory/pro-oxidant environment and symptoms of diabetic bladder dysfunction. Our results show increased cellular senescence in the urothelial layer of the bladder; however, whether this phenomenon is the cause or effect of DBD is unknown. The urothelial layer of the bladder is made up of transitional epithelia specialized to contract and expand with demand and plays an active role in transmission by modulating afferent activity. Transition from normal functioning urothelial cells to secretory senescence cells would not only disrupt the barrier function of this layer but may result in altered signaling and sensation of bladder fullness; dysfunction of this layer is known to result in symptoms of frequency and urgency. Future DBD therapeutics may benefit from targeting and preventing early transition of urothelial cells to senescent cells.
Keywords: diabetic bladder; diabetic cystopathy; lower urinary tract; senescence; urothelium.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

