SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer (2018)
- PMID: 30565083
- PMCID: PMC6339676
- DOI: 10.1007/s12094-018-02002-w
SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer (2018)
Abstract
Colorectal cancer (CRC) is the second cause of cancer death in Spain, the objective of this guide published by the Spanish Society of Medical Oncology is to develop a consensus for the diagnosis and management of metastatic disease. The optimal treatment strategy for patients with metastatic CRC should be discussed in a multidisciplinary expert team to select the most appropriate treatment, and integrate systemic treatment and other options such as surgery and ablative techniques depending on the characteristics of the tumour, the patient and the location of the disease and metastases.
Keywords: Ablative treatments; Chemotherapy; Colorectal cancer; Frail patients; Metastases; Surgery; Targeted agents.
Conflict of interest statement
Conflict of interest
MAGE reports financial support from Angem, Kyowakirin, Merck, Roche and Servier, outside the submitted work. JG reports advisory board from Amgen, Bayer, Ipsen, Lilly, Merck, Roche and Sanofi. Speaker honoraria from Amgen, Ipsen and Lilly and Subsidies from Amgen and Novartis, outside the submitted work. EGF reports Advisory board from Roche, Amgen, Merck, Bayer, Servier and Sanofi, outside the submitted work. JM reports Grants non-remunerated (clinical trial principal investigator) from Merck and Amgen, and grants non-remunerated (Project lead principal investigator) from Nanostring and Biocartis. Also reports personal fees from Sirtex, Pierre-Fabre, Shire, Astrazeneca, Bayer, Servier, Sanofi and Roche, outside the submitted work. DP reports personal fees and non-financial support from Amgen, personal fees from Sanofi, personal fees and non financial support from Merck Serono, personal fees and non-financial support from Servier, personal fees and non-financial support from F. Hoffmann-La Roche Ltd, outside the submitted work. JS reports personal fees from Merck, Ipsen, Roche, Lilly, Shire, Sanofi, Servier, Amgen, Bayer, Celgene, Bristol-Myers Squibb and Pfizer and non-financial support from Merck and Ipsen, outside the submitted work. JA reports consultant or advisory role from Bayer, Servier, Merck, Sanofi, Amgen, Sirtex, Roche and Celgene, outside the submitted work. MB reports personal fees and non-financial support from Roche, Amgen, Sanofi, Merck, Abbvie, Sysmex, outside the submitted work. JF reports grants from Merck and advisory board from Amgen, Ipsen, Eisai, Celgene, Novartris, outside the submitted work. RV reports personal fees from Sanofi, Roche, Msd, Merck, Amgen and Bristol-Myers Squibb, outside the submitted work.
Ethical approval
The current study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
For this type of study formal consent is not required.
Figures

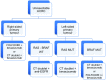
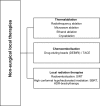
References
-
- - Amin, MB, Greene F, Edge S, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual (ed. 8th edition). New York: Springer; 2016.
-
- Chibaudel B, Bonnetain F, Tournigand C, Bengrine-Lefevre L, Teixeira L, Artru P, et al. Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR Study. Oncologist. 2011;16:1228–1238. doi: 10.1634/theoncologist.2011-0039. - DOI - PMC - PubMed
-
- Arnold D, Lueza B, Douillard J-Y, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–1729. doi: 10.1093/annonc/mdx175. - DOI - PMC - PubMed

