Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors
- PMID: 30565258
- PMCID: PMC6438731
- DOI: 10.1111/jnc.14646
Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors
Abstract
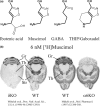
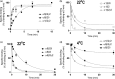
Muscimol, the major psychoactive ingredient in the mushroom Amanita muscaria, has been regarded as a universal non-selective GABA-site agonist. Deletion of the GABAA receptor (GABAA R) δ subunit in mice (δKO) leads to a drastic reduction in high-affinity muscimol binding in brain sections and to a lower behavioral sensitivity to muscimol than their wild type counterparts. Here, we use forebrain and cerebellar brain homogenates from WT and δKO mice to show that deletion of the δ subunit leads to a > 50% loss of high-affinity 5 nM [3 H]muscimol-binding sites despite the relatively low abundance of δ-containing GABAA Rs (δ-GABAA R) in the brain. By subtracting residual high-affinity binding in δKO mice and measuring the slow association and dissociation rates we show that native δ-GABAA Rs in WT mice exhibit high-affinity [3 H]muscimol-binding sites (KD ~1.6 nM on α4βδ receptors in the forebrain and ~1 nM on α6βδ receptors in the cerebellum at 22°C). Co-expression of the δ subunit with α6 and β2 or β3 in recombinant (HEK 293) expression leads to the appearance of a slowly dissociating [3 H]muscimol component. In addition, we compared muscimol currents in recombinant α4β3δ and α4β3 receptors and show that δ subunit co-expression leads to highly muscimol-sensitive currents with an estimated EC50 of around 1-2 nM and slow deactivation kinetics. These data indicate that δ subunit incorporation leads to a dramatic increase in GABAA R muscimol sensitivity. We conclude that biochemical and behavioral low-dose muscimol selectivity for δ-subunit-containing receptors is a result of low nanomolar-binding affinity on δ-GABAA Rs.
Keywords: GABAA receptors; affinity; association; binding; dissociation; muscimol.
© 2018 International Society for Neurochemistry.
Conflict of interest statement
This study was supported by grants from the Finnish Foundation for Alcohol Studies (AJA, MU‐O) and NIH grant AA021213 to MW. The authors have no conflict of interest to declare.
Figures



References
-
- Adkins C. E., Pillai G. V., Kerby J., Bonnert T. P., Haldon C., McKernan R. M., Gonzalez J. E., Oades K., Whiting P. J. and Simpson P. B. (2001) α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer‐derived measurements of membrane potential. J. Biol. Chem. 276, 38934–38939. - PubMed
-
- Agey M. W. and Dunn S. M. (1989) Kinetics of [3H]muscimol binding to the GABAA receptor in bovine brain membranes. Biochemistry 28, 4200–4208. - PubMed
-
- Barrera N. P., Betts J., You H., Henderson R. M., Martin I. L., Dunn S. M. and Edwardson J. M. (2008) Atomic force microscopy reveals the stoichiometry and subunit arrangement of the α4β3δ GABAA receptor. Mol. Pharmacol. 73, 960–967. - PubMed
-
- Boehm S. L., 2nd , Homanics G. E., Blednov Y. A. and Harris R. A. (2006) Delta‐subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7‐tetrahydroisoxazolo‐[5,4‐c]pyridin‐3‐ol. Eur. J. Pharmacol. 541, 158–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

