Downsizing the molecular spring of the giant protein titin reveals that skeletal muscle titin determines passive stiffness and drives longitudinal hypertrophy
- PMID: 30565562
- PMCID: PMC6300359
- DOI: 10.7554/eLife.40532
Downsizing the molecular spring of the giant protein titin reveals that skeletal muscle titin determines passive stiffness and drives longitudinal hypertrophy
Abstract
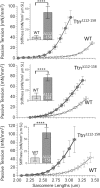
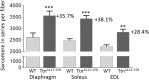
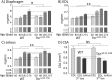
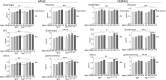
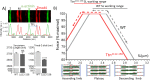
Titin, the largest protein known, forms an elastic myofilament in the striated muscle sarcomere. To establish titin's contribution to skeletal muscle passive stiffness, relative to that of the extracellular matrix, a mouse model was created in which titin's molecular spring region was shortened by deleting 47 exons, the TtnΔ112-158 model. RNA sequencing and super-resolution microscopy predicts a much stiffer titin molecule. Mechanical studies with this novel mouse model support that titin is the main determinant of skeletal muscle passive stiffness. Unexpectedly, the in vivo sarcomere length working range was shifted to shorter lengths in TtnΔ112-158 mice, due to a ~ 30% increase in the number of sarcomeres in series (longitudinal hypertrophy). The expected effect of this shift on active force generation was minimized through a shortening of thin filaments that was discovered in TtnΔ112-158 mice. Thus, skeletal muscle titin is the dominant determinant of physiological passive stiffness and drives longitudinal hypertrophy.
Editorial note: This article has been through an editorial process in which the authors decide how to respond to the issues raised during peer review. The Reviewing Editor's assessment is that all the issues have been addressed (see decision letter).
Keywords: biomechanics; elasticity; mouse; muscle; myofilament function; passive stiffness; physics of living systems; titinopathies.
© 2018, Brynnel et al.
Conflict of interest statement
AB, YH, BK, JL, MA, JK, RV, JG, JS, JS, CO, HG No competing interests declared
Figures










Similar articles
-
Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness.J Muscle Res Cell Motil. 2003;24(2-3):175-89. doi: 10.1023/a:1026053530766. J Muscle Res Cell Motil. 2003. PMID: 14609029
-
Deleting Titin's C-Terminal PEVK Exons Increases Passive Stiffness, Alters Splicing, and Induces Cross-Sectional and Longitudinal Hypertrophy in Skeletal Muscle.Front Physiol. 2020 May 29;11:494. doi: 10.3389/fphys.2020.00494. eCollection 2020. Front Physiol. 2020. PMID: 32547410 Free PMC article.
-
Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics.Biophys J. 1996 Jan;70(1):430-42. doi: 10.1016/S0006-3495(96)79586-3. Biophys J. 1996. PMID: 8770219 Free PMC article.
-
Physiological functions of the giant elastic protein titin in mammalian striated muscle.J Physiol Sci. 2008 Jun;58(3):151-9. doi: 10.2170/physiolsci.RV005408. Epub 2008 May 15. J Physiol Sci. 2008. PMID: 18477421 Review.
-
Stretching the story of titin and muscle function.J Biomech. 2023 May;152:111553. doi: 10.1016/j.jbiomech.2023.111553. Epub 2023 Mar 23. J Biomech. 2023. PMID: 36989971 Review.
Cited by
-
Investigating Passive Muscle Mechanics With Biaxial Stretch.Front Physiol. 2020 Aug 20;11:1021. doi: 10.3389/fphys.2020.01021. eCollection 2020. Front Physiol. 2020. PMID: 32973555 Free PMC article.
-
Re‑examining the mechanism of eccentric exercise‑induced skeletal muscle damage from the role of the third filament, titin (Review).Biomed Rep. 2023 Dec 1;20(1):14. doi: 10.3892/br.2023.1703. eCollection 2024 Jan. Biomed Rep. 2023. PMID: 38124762 Free PMC article. Review.
-
Tension-driven multi-scale self-organisation in human iPSC-derived muscle fibers.Elife. 2022 Aug 3;11:e76649. doi: 10.7554/eLife.76649. Elife. 2022. PMID: 35920628 Free PMC article.
-
Training Induced Changes to Skeletal Muscle Passive Properties Are Evident in Both Single Fibers and Fiber Bundles in the Rat Hindlimb.Front Physiol. 2020 Aug 12;11:907. doi: 10.3389/fphys.2020.00907. eCollection 2020. Front Physiol. 2020. PMID: 32903515 Free PMC article.
-
History-dependence of muscle slack length in humans: effects of contraction intensity, stretch amplitude, and time.J Appl Physiol (1985). 2020 Oct 1;129(4):957-966. doi: 10.1152/japplphysiol.00106.2020. Epub 2020 Sep 3. J Appl Physiol (1985). 2020. PMID: 32881621 Free PMC article.
References
-
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circulation Research. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. - DOI - PubMed
-
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. The Journal of Experimental Biology. 2001;204:1529–1536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

