Minimally Invasive and Regenerative Therapeutics
- PMID: 30565732
- PMCID: PMC6709364
- DOI: 10.1002/adma.201804041
Minimally Invasive and Regenerative Therapeutics
Abstract
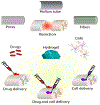
Advances in biomaterial synthesis and fabrication, stem cell biology, bioimaging, microsurgery procedures, and microscale technologies have made minimally invasive therapeutics a viable tool in regenerative medicine. Therapeutics, herein defined as cells, biomaterials, biomolecules, and their combinations, can be delivered in a minimally invasive way to regenerate different tissues in the body, such as bone, cartilage, pancreas, cardiac, skeletal muscle, liver, skin, and neural tissues. Sophisticated methods of tracking, sensing, and stimulation of therapeutics in vivo using nano-biomaterials and soft bioelectronic devices provide great opportunities to further develop minimally invasive and regenerative therapeutics (MIRET). In general, minimally invasive delivery methods offer high yield with low risk of complications and reduced costs compared to conventional delivery methods. Here, minimally invasive approaches for delivering regenerative therapeutics into the body are reviewed. The use of MIRET to treat different tissues and organs is described. Although some clinical trials have been performed using MIRET, it is hoped that such therapeutics find wider applications to treat patients. Finally, some future perspective and challenges for this emerging field are highlighted.
Keywords: biomaterials; biomolecules; delivery; minimally invasive; scaffolds; tissue regeneration.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

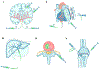

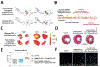








References
-
- He W, Goodkind D, Kowal P, An Aging World: 2015, International Population Report No. P95/16–1, United States Census Bureau, Washington, DC, USA: 2016.
-
- Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li R-K, Caldarone CA, Radisic M, Nature Materials 2017, 16, 1038. - PubMed
-
- Krettek C, Schandelmaier P, Nliclau T, Tscherne H, Injury 1997, 28, A20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA214411/CA/NCI NIH HHS/United States
- R01 HL140951/HL/NHLBI NIH HHS/United States
- R01 EB023052/EB/NIBIB NIH HHS/United States
- R01 AR066193/AR/NIAMS NIH HHS/United States
- EB023052/NH/NIH HHS/United States
- R01 EB024403/EB/NIBIB NIH HHS/United States
- EB021857/NH/NIH HHS/United States
- CA214411/NH/NIH HHS/United States
- EB024403/NH/NIH HHS/United States
- R21 EB022403/EB/NIBIB NIH HHS/United States
- HL137193/NH/NIH HHS/United States
- AR066193/NH/NIH HHS/United States
- R01 AR057837/AR/NIAMS NIH HHS/United States
- AR057837/NH/NIH HHS/United States
- R01 AR073135/AR/NIAMS NIH HHS/United States
- EB022403/NH/NIH HHS/United States
- FA9550-15-1-0273/Air Force Office of Sponsored Research
- R01 EB021857/EB/NIBIB NIH HHS/United States
- R01 HL137193/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

