ERβ agonist alters RNA splicing factor expression and has a longer window of antidepressant effectiveness than estradiol after long-term ovariectomy
- PMID: 30565903
- PMCID: PMC6306290
- DOI: 10.1503/jpn.170199
ERβ agonist alters RNA splicing factor expression and has a longer window of antidepressant effectiveness than estradiol after long-term ovariectomy
Abstract
Background: Estrogen therapy (ET), an effective treatment for perimenopausal depression, often fails to ameliorate symptoms when initiated late after the onset of menopause. Our previous work has suggested that alternative splicing of RNA might mediate these differential effects of ET.
Methods: Female Sprague–Dawley rats were treated with estradiol (E2) or vehicle 6 days (early ET) or 180 days (late ET) after ovariectomy (OVX). We investigated the differential expression of RNA splicing factors and tryptophan hydroxylase 2 (TPH2) protein using a customized RT2 Profiler PCR Array, reverse-transcription polymerase chain reaction, immunoprecipitation and behaviour changes in clinically relevant early and late ET.
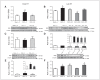
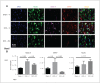
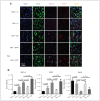
Results: Early ET, but not late ET, prolonged swimming time in the forced swim test and reduced anxiety-like behaviours in the elevated plus maze. It reversed OVX-increased (SFRS7 and SFRS16) or OVX-decreased (ZRSR2 and CTNNB1) mRNA levels of splicing factors and ERβ splicing changes in the brains of OVX rats. Early ET, but not late ET, also increased the expression of TPH2 and decreased monoamine oxidase A levels in the dorsal raphe in the brains of OVX rats. In late ET, only diarylpropionitrile (an ERβ-specific agonist) achieved similar results — not E2 (an ERα and ERβ agonist) or propylpyrazoletriol (an ERα-specific agonist).
Limitations: Our experimental paradigm mimicked early and late ET in the clinical setting, but the contribution of age and OVX might be difficult to distinguish.
Conclusion: These findings suggest that ERβ alternative splicing and altered responses in the regulatory system for serotonin may mediate the antidepressant efficacy of ET associated with the timing of therapy initiation. It is likely that ERβ-specific ligands would be effective estrogen-based antidepressants late after the onset of menopause.
© 2019 Joule Inc. or its licensors
Conflict of interest statement
J. Meyer reports grants from Janssen, outside the submitted work. In addition, he has patents on a brain marker and blood markers of MAO-A for predicting mood disorder and on a dietary supplement to prevent postpartum depression and sad mood during high MAO-A states. No other competing interests declared.
Figures


Similar articles
-
Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei.Neuroscience. 2009 Oct 6;163(2):705-18. doi: 10.1016/j.neuroscience.2009.06.046. Epub 2009 Jun 23. Neuroscience. 2009. PMID: 19559077 Free PMC article.
-
Antidepressant-like effects of Xiaochaihutang in perimenopausal mice.J Ethnopharmacol. 2020 Feb 10;248:112318. doi: 10.1016/j.jep.2019.112318. Epub 2019 Oct 17. J Ethnopharmacol. 2020. PMID: 31629860
-
Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress.Endocrinology. 2009 Apr;150(4):1817-25. doi: 10.1210/en.2008-1355. Epub 2008 Dec 12. Endocrinology. 2009. PMID: 19074580 Free PMC article.
-
ERβ activation improves nonylphenol-induced depression and neurotransmitter secretion disruption via the TPH2/5-HT pathway.Ecotoxicol Environ Saf. 2024 Jul 15;280:116521. doi: 10.1016/j.ecoenv.2024.116521. Epub 2024 Jun 8. Ecotoxicol Environ Saf. 2024. PMID: 38850708
-
Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines.Neurobiol Learn Mem. 2010 Nov;94(4):488-98. doi: 10.1016/j.nlm.2010.08.016. Epub 2010 Sep 7. Neurobiol Learn Mem. 2010. PMID: 20828630 Free PMC article.
Cited by
-
Oral contraceptives and the serotonin 4 receptor: a molecular brain imaging study in healthy women.Acta Psychiatr Scand. 2020 Oct;142(4):294-306. doi: 10.1111/acps.13211. Epub 2020 Jul 21. Acta Psychiatr Scand. 2020. PMID: 33314049 Free PMC article.
-
MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model.Cells. 2019 Sep 13;8(9):1081. doi: 10.3390/cells8091081. Cells. 2019. PMID: 31540304 Free PMC article.
References
-
- Parker G, Fletcher K, Paterson A, et al. Gender differences in depression severity and symptoms across depressive sub-types. J Affect Disord. 2014;167:351–7. - PubMed
-
- Soares CN. Depression in peri- and postmenopausal women: prevalence, pathophysiology and pharmacological management. Drugs Aging. 2013;30:677–85. - PubMed
-
- Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–90. - PubMed
-
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
