Synthesis, Characterization and in vitro Studies of a Cathepsin B-Cleavable Prodrug of the VEGFR Inhibitor Sunitinib
- PMID: 30566287
- PMCID: PMC6391952
- DOI: 10.1002/cbdv.201800520
Synthesis, Characterization and in vitro Studies of a Cathepsin B-Cleavable Prodrug of the VEGFR Inhibitor Sunitinib
Abstract
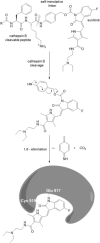
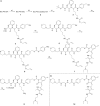
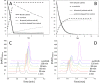
Since several decades, the prodrug concept has raised considerable interest in cancer research due to its potential to overcome common problems associated with chemotherapy. However, for small-molecule tyrosine kinase inhibitors, which also cause severe side effects, hardly any strategies to generate prodrugs for therapeutic improvement have been reported so far. Here, we present the synthesis and biological investigation of a cathepsin B-cleavable prodrug of the VEGFR inhibitor sunitinib. Cell viability assays and Western blot analyses revealed, that, in contrast to the non-cathepsin B-cleavable reference compound, the prodrug shows activity comparable to the original drug sunitinib in the highly cathepsin B-expressing cell lines Caki-1 and RU-MH. Moreover, a cathepsin B cleavage assay confirmed the desired enzymatic activation of the prodrug. Together, the obtained data show that the concept of cathepsin B-cleavable prodrugs can be transferred to the class of targeted therapeutics, allowing the development of optimized tyrosine kinase inhibitors for the treatment of cancer.
Keywords: cathepsin B; inhibitors; prodrugs; sunitinib; tyrosine kinase inhibitor; vascular endothelial growth factor receptor (VEGFR).
© 2019 The Authors. Published by Wiley-VHCA AG, Zurich, Switzerland.
Figures


References
-
- N. J. Harper, ‘Drug Latentiation’, J. Med. Pharm. Chem 1959, 1, 467–500. - PubMed
-
- F. Kratz, I. A. Müller, C. Ryppa, A. Warnecke, ‘Prodrug Strategies in Anticancer Chemotherapy’, ChemMedChem 2008, 3, 20–53. - PubMed
-
- W. R. Wilson, M. P. Hay, ‘Targeting hypoxia in cancer therapy’, Nat. Rev. Cancer 2011, 11, 393–410. - PubMed
-
- J. Vandooren, G. Opdenakker, P. M. Loadman, D. R. Edwards, ‘Proteases in cancer drug delivery’, Adv. Drug Delivery Rev 2016, 97, 144–155. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

