Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis
- PMID: 30566596
- PMCID: PMC6370266
- DOI: 10.1093/advances/nmy076
Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis
Abstract
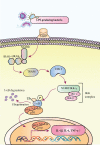
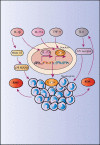
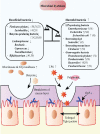
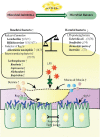
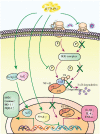
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis is one of the main causes of intestinal mucositis. Cases of bacterial translocation into peripheral blood and subsequent sepsis occur as a result of dysfunction in the intestinal barrier. Evidence from recent studies depicts the characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis, which creates an imbalance between beneficial and harmful bacteria in the gut. Decreases in beneficial bacteria can lead to a weakening of the resistance of the gut to harmful bacteria, resulting in robust activation of proinflammatory signaling pathways. For example, lipopolysaccharide (LPS)-producing bacteria activate the nuclear transcription factor-κB signaling pathway through binding with Toll-like receptor 4 on stressed epithelial cells, subsequently leading to secretion of proinflammatory cytokines. Nevertheless, various studies have found that the omega-3 (n-3) polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid and eicosapentaenoic acid can reverse intestinal microbial dysbiosis by increasing beneficial bacteria species, including Lactobacillus, Bifidobacterium, and butyrate-producing bacteria, such as Roseburia and Coprococcus. In addition, the n-3 PUFAs decrease the proportions of LPS-producing and mucolytic bacteria in the gut, and they can reduce inflammation as well as oxidative stress. Importantly, the n-3 PUFAs also exert anticancer effects in colorectal cancers. In this review, we summarize the characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis and introduce the contributions of dysbiosis to the pathogenesis of intestinal mucositis. Next, we discuss how n-3 PUFAs could alleviate chemotherapy- or radiotherapy-related intestinal microbial dysbiosis. This review provides new insights into the clinical administration of n-3 PUFAs for the management of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis.
Figures
References
-
- Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007;109(5):820–31. - PubMed

