Incomplete scleral penetration of dexamethasone (Ozurdex) intravitreal implant
- PMID: 30567246
- PMCID: PMC6301585
- DOI: 10.1136/bcr-2018-227055
Incomplete scleral penetration of dexamethasone (Ozurdex) intravitreal implant
Abstract
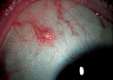
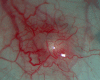
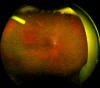
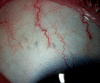
Ozurdex (Allergan, Irvine, California, USA) is a biodegradable sustained release intravitreal implant containing 0.7 mg dexamethasone in a solid polymer drug delivery system. In the UK, it is approved for use in patients with macular oedema secondary to retinal vein occlusion, diabetic maculopathy and non-infectious uveitis. Although the implant is meant to be injected into the vitreous cavity, it can be inadvertently injected into the crystalline lens. This can also migrate into the anterior chamber, under altered anatomical conditions of the anterior segment. We report a case of incompletely penetrated dexamethasone implant, in a patient undergoing treatment for macular oedema secondary to retinal vein occlusion. The partially penetrated implant was managed conservatively with a good outcome.
Keywords: macula; retina.
© BMJ Publishing Group Limited 2018. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
SD-OCT pattern of retinal venous occlusion with cystoid macular edema treated with Ozurdex®.Eur J Ophthalmol. 2011 Sep-Oct;21(5):631-6. doi: 10.5301/EJO.2011.7428. Eur J Ophthalmol. 2011. PMID: 21500185
-
Evolution of vitreomacular traction following the use of the dexamethasone intravitreal implant (Ozurdex) in the treatment of macular edema secondary to central retinal vein occlusion.J Ocul Pharmacol Ther. 2012 Oct;28(5):547-9. doi: 10.1089/jop.2011.0184. Epub 2012 Apr 26. J Ocul Pharmacol Ther. 2012. PMID: 22537269
-
Intracrystalline Ozurdex®: therapeutic effect maintained for 18 months.Int Ophthalmol. 2019 Jan;39(1):207-211. doi: 10.1007/s10792-017-0780-3. Epub 2017 Nov 30. Int Ophthalmol. 2019. PMID: 29192395
-
OZURDEX® (Dexamethasone Intravitreal Implant) 0.7 mg as Initial Therapy in Pseudophakic Patients With Diabetic Macular Edema, Macular Edema Following Retinal Vein Occlusion, and Noninfectious Posterior Segment Uveitis: A Case-Based Discussion.Retina. 2019 Jun;39 Suppl 1:S1-S28. doi: 10.1097/01.iae.0000574292.81105.b2. Retina. 2019. PMID: 31206423 Review. No abstract available.
-
Dexamethasone intravitreal implant (OZURDEX®) for macular edema secondary to noninfectious uveitis: a review of the literature.Ther Deliv. 2019 Jun 1;10(6):343-351. doi: 10.4155/tde-2019-0024. Epub 2019 Jun 11. Ther Deliv. 2019. PMID: 31184554 Review.
Cited by
-
Intravitreal Dexamethasone Implant as a Sustained Release Drug Delivery Device for the Treatment of Ocular Diseases: A Comprehensive Review of the Literature.Pharmaceutics. 2020 Jul 26;12(8):703. doi: 10.3390/pharmaceutics12080703. Pharmaceutics. 2020. PMID: 32722556 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
