Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents
- PMID: 30567327
- PMCID: PMC6321601
- DOI: 10.3390/molecules23123345
Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents
Abstract
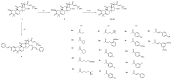
Cucurbitacin B shows potent activity against tumor cells, but its high toxicity limits its application in the clinic. A series of cucurbitacin B derivatives was synthesized and evaluated for their anti-hepatocellular carcinoma (HCC) activities against the HepG-2 cell line. These compounds were also tested for their toxicity against the L-O2 normal cell line. The compound with the most potential, 10b, exhibited potent activity against the HepG-2 cell line with an IC50 value of 0.63 μM. Moreover, compound 10b showed the highest TI value (4.71), which is a 14.7-fold improvement compared to its parent compound cucurbitacin B. A preliminary molecular mechanism study of 10b indicated that 10b could inhibit P-STAT3 to induce the activation of mitochondrial apoptotic pathways. An in vivo acute toxicity study indicated that the compound 10b has preferable safety and tolerability compared with cucurbitacin B. These findings indicate that compound 10b might be considered as a lead compound for exploring effective anti-HCC drugs.
Keywords: Anti-hepatocellular carcinoma; Cucurbitacin B; Derivative; Synthesis; Toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B.Cancer Chemother Pharmacol. 2009 Mar;63(4):635-42. doi: 10.1007/s00280-008-0780-0. Epub 2008 Jun 3. Cancer Chemother Pharmacol. 2009. PMID: 18521604
-
Cucurbitacin I exerts its anticancer effects by inducing cell cycle arrest via the KAT2a-ube2C/E2F1 pathway and inhibiting HepG2-induced macrophage M2 polarization.Biochem Biophys Res Commun. 2024 Dec 17;738:150508. doi: 10.1016/j.bbrc.2024.150508. Epub 2024 Aug 5. Biochem Biophys Res Commun. 2024. PMID: 39151295
-
Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling pathway, enhances the chemosensitivity of laryngeal squamous cell carcinoma cells to cisplatin.Eur J Pharmacol. 2010 Sep 1;641(1):15-22. doi: 10.1016/j.ejphar.2010.04.062. Epub 2010 May 17. Eur J Pharmacol. 2010. PMID: 20483353
-
Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration.Cancer Lett. 2010 Aug 1;294(1):118-24. doi: 10.1016/j.canlet.2010.01.029. Epub 2010 Feb 11. Cancer Lett. 2010. PMID: 20153103
-
Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities.Molecules. 2024 Sep 4;29(17):4193. doi: 10.3390/molecules29174193. Molecules. 2024. PMID: 39275042 Free PMC article. Review.
Cited by
-
Analgesic Effect of SH003 and Trichosanthes kirilowii Maximowicz in Paclitaxel-Induced Neuropathic Pain in Mice.Curr Issues Mol Biol. 2022 Jan 31;44(2):718-730. doi: 10.3390/cimb44020050. Curr Issues Mol Biol. 2022. PMID: 35723335 Free PMC article.
-
Metabolic fate of the natural anticancer agent cucurbitacin B: an LC-MS/MS-enabled profiling of its major phase I and II conjugates in vivo.Anal Bioanal Chem. 2024 Dec;416(29):7043-7062. doi: 10.1007/s00216-024-05608-y. Epub 2024 Oct 23. Anal Bioanal Chem. 2024. PMID: 39441433
-
Progress Toward the Asymmetric de Novo Synthesis of Lanostanes: A Counter Biomimetic Cucurbitane-to-Lanostane Type Transformation.Tetrahedron. 2023 Jul 17;141:133498. doi: 10.1016/j.tet.2023.133498. Epub 2023 Jun 2. Tetrahedron. 2023. PMID: 37637188 Free PMC article.
-
Pharmacokinetics and Biological Activity of Cucurbitacins.Pharmaceuticals (Basel). 2022 Oct 26;15(11):1325. doi: 10.3390/ph15111325. Pharmaceuticals (Basel). 2022. PMID: 36355498 Free PMC article. Review.
-
Asymmetric De Novo Synthesis of a Cucurbitane Triterpenoid: Total Synthesis of Octanorcucurbitacin B.J Am Chem Soc. 2022 May 18;144(19):8493-8497. doi: 10.1021/jacs.2c03109. Epub 2022 May 9. J Am Chem Soc. 2022. PMID: 35533213 Free PMC article.
References
-
- Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

