Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid
- PMID: 30567370
- PMCID: PMC6315479
- DOI: 10.3390/nano8121068
Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid
Abstract
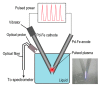
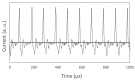
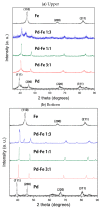
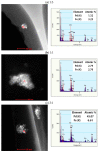
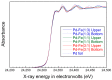
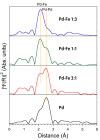
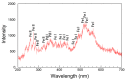
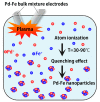
We synthesized Pd-Fe series nanoparticles in solid solution using pulsed plasma in liquid with Pd-Fe bulk mixture electrodes. The Pd-Fe atomic percent ratios were 1:3, 1:1, and 3:1, and the particle size was measured to be less than 10 nm by high-resolution transmission electron microscopy (HR-TEM). The nanoparticles showed face-centered cubic structure. The lattice parameter increased with increasing Pd content and followed Vegard's law, and energy-dispersive X-ray spectra were consistent with the ratios of the starting samples, which showed a solid solution state. The solid solution structure and local structure were confirmed by HR-TEM and X-ray absorption fine structure.
Keywords: Pd-Fe alloy; nanoparticle; pulsed plasma in liquid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gelatt C.D., Ehrenreich H., Weiss J.A. Transition-metal hydrides: Electronic structure and the heats of formation. Phys. Rev. B. 1978;17:1940–1957. doi: 10.1103/PhysRevB.17.1940. - DOI
-
- Vuillemin J.J., Priestly M.G. De Haas-Van Alphen effect and Fermi surface in palladium. Phys. Rev. Lett. 1965;14:307–309. doi: 10.1103/PhysRevLett.14.307. - DOI
-
- Ke X., Cui G.-F., Shen P.-K. Stability of Pd-Fe Alloy Catalysts. Wuli Huaxue Xuebao Acta Phys. Chim. Sin. 2009;25:213–217.
LinkOut - more resources
Full Text Sources

