Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: a pilot study
- PMID: 30567535
- PMCID: PMC6299992
- DOI: 10.1186/s12906-018-2403-6
Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: a pilot study
Abstract
Background: (Trans)-3,7-Dimethyl-2,6-octadien-1-ol, commonly called geraniol (Ge-OH), is an acyclic monoterpene alcohol with well-known anti-inflammatory and antimicrobial properties. Ge-OH is a non-toxic compound classified as Generally Recognized As Safe (GRAS) by the US Food and Drug Administration and the European Food Security Agency.
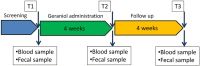
Methods: Ge-OH was orally administered at a maximum daily dose of 8 mg kg(- 1) body weight for four weeks in a delayed release formulation capable of reaching the colon. Fecal microbiota and blood cytokines were analyzed before and after Ge-OH treatment, as well as IBS symptomatology by using Visual Analogue Scale (VAS-IBS).
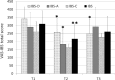
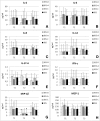
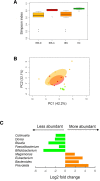
Results: The results show that orally administered Ge-OH is a powerful modulator of the intestinal microbial ecosystem, capable of leading to increased relative abundances of Collinsella and especially Faecalibacterium, a well-known health-promoting butyrate producer consistently found to be decreased in IBS patients. Moreover, Ge-OH strongly improved the clinical symptoms of colitis by significantly reducing the score recorded by the VAS-IBS questionnaire. Clinical improvement was associated with a significant reduction in the circulating MIP-1β, a chemokine found to be increased in several IBS patients.
Conclusion: Ge-OH could be a powerful component for food supplement targeted to the treatment of IBS patients.
Trial registration: ISRCTN47041881 , retrospectively registered on 19th July 2018.
Keywords: Dysbiosis; Geraniol; Inflammation; Irritable bowel syndrome (IBS); Microbiota.
Conflict of interest statement
Ethics approval and consent to participate
Patients were informed of the full nature and purpose of the study, and provided written informed consent before entering the trial. The study was conducted in conformity with the principles of Declaration of Helsinki and Good Clinical Practice. The study was approved by the Ethics Committee of the AOU Policlinico S. Orsola-Malpighi; CE code 100/2013/U/Sper and by the Ethics Committee of the ASST Spedali Civili di Brescia; CE code NP2047.
Consent for publication
All patients gave explicit written consent for data publication in aggregated form.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Holten KB, Wetherington A, Bankston L. Diagnosing the patient with abdominal pain and altered bowel habits: is it irritable bowel syndrome? Am Fam Physician. 2003;67:2157–2162. - PubMed
-
- Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: a multinational consensus document on functional gastrointestinal disorders. Gut. 1999;45:SII II1–II81. doi: 10.1136/gut.45.2.181. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

