Structured diet and exercise guidance in pregnancy to improve health in women and their offspring: study protocol for the Be Healthy in Pregnancy (BHIP) randomized controlled trial
- PMID: 30567604
- PMCID: PMC6299965
- DOI: 10.1186/s13063-018-3065-x
Structured diet and exercise guidance in pregnancy to improve health in women and their offspring: study protocol for the Be Healthy in Pregnancy (BHIP) randomized controlled trial
Abstract
Background: Evidence from epidemiological and animal studies support the concept of programming fetal, neonatal, and adult health in response to in utero exposures such as maternal obesity and lifestyle variables. Excess gestational weight gain (GWG), maternal physical activity, and sub-optimal and excess nutrition during pregnancy may program the offspring's risk of obesity. Maternal intake of dairy foods rich in high-quality proteins, calcium, and vitamin D may influence later bone health status. Current clinical practice guidelines for managing GWG are not founded on randomized trials and lack specific "active intervention ingredients." The Be Healthy in Pregnancy (BHIP) study is a randomized controlled trial (RCT) designed to test the effectiveness of a novel structured and monitored Nutrition + Exercise intervention in pregnant women of all pre-pregnancy weight categories (except extreme obesity), delivered through prenatal care in community settings (rather than in hospital settings), on the likelihood of women achieving recommended GWG and a benefit to bone status of offspring and mother at birth and six months postpartum.
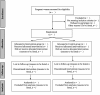
Methods: The BHIP study is a two-site RCT that will recruit up to 242 participants aged > 18 years at 12-17 weeks of gestation. After baseline measures, participants are randomized to either a structured and monitored Nutrition + Exercise (intervention) or usual care (control) program for the duration of their pregnancy. The primary outcome of the study is the percent of women who achieve GWG within the Institute of Medicine (IOM) guidelines. The secondary outcomes include: (1) maternal bone status via blood bone biomarkers during pregnancy; (2) infant bone status in cord blood; (3) mother and infant bone status measured by dual-energy absorptiometry scanning (DXA scan) at six months postpartum; (4) other measures including maternal blood pressure, blood glucose and lipid profiles, % body fat, and postpartum weight retention; and (5) infant weight z-scores and fat mass at six months of age.
Discussion: If effective, this RCT will generate high-quality evidence to refine the nutrition guidelines during pregnancy to improve the likelihood of women achieving recommended GWG. It will also demonstrate the importance of early nutrition on bone health in the offspring.
Trial registration: ClinicalTrials.gov, NCT01689961 Registered on 21 September 2012.
Keywords: Bone; Dairy foods; Developmental origins of health and disease; Exercise; Gestational weight gain; Infancy; Nutrition; Pregnancy; Proteins; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
After independent, full, external peer review the study protocol and subsequent amendments have been approved by the Hamilton Integrated Research Ethics Board (REB Project no. 12–469, McMaster University and its associated sites, Hamilton, ON), the Joseph Brant Hospital Research Ethics Committee (Project titled “Be Healthy in Pregnancy (B-HIP): An RCT to study nutrition and exercise approaches for healthy pregnancy,” Joseph Brant Hospital, Burlington, ON), and the Health Sciences Research Ethics Board (HSREB File no. 103272, Western University and its associated sites, London, ON). This study is being conducted in compliance with the protocol, Good Clinical Practice and the applicable regulatory requirements.
The Research Ethics boards have approved changes to the protocol since the inception of the clinical trial. All changes were communicated to relevant parties and modified on the trial registries. Of significance, the inclusion criteria was modified: increasing BMI from 30 to 40 kg/m2 to capture a representative sample of the community who presents an important percentage of obese women; participants have to be randomized to group allocation before 17 weeks 6 days (initially set at 11 weeks 6 days) to maximize participant enrolment while ensuring an intervention period of at least 20 weeks. Further, two groups originally excluded were added to the inclusion criteria: women whose pregnancy is a result of in vitro fertilization and women currently breastfeeding from a previous pregnancy. Exclusion criteria were also modified to redefine exclusion of participants with a prenatal depression score > 12 (rather than the initial score of 10) on the validated Edinburgh Depression scale as that is indicative of severe depression and should be referred for treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.