Platelet abnormalities in Huntington's disease
- PMID: 30567722
- PMCID: PMC6518476
- DOI: 10.1136/jnnp-2018-318854
Platelet abnormalities in Huntington's disease
Abstract
Huntington's disease (HD) is a hereditary disorder that typically manifests in adulthood with a combination of motor, cognitive and psychiatric problems. The pathology is caused by a mutation in the huntingtin gene which results in the production of an abnormal protein, mutant huntingtin (mHtt). This protein is ubiquitously expressed and known to confer toxicity to multiple cell types. We have recently reported that HD brains are also characterised by vascular abnormalities, which include changes in blood vessel density/diameter as well as increased blood-brain barrier (BBB) leakage.
Objectives: Seeking to elucidate the origin of these vascular and BBB abnormalities, we studied platelets that are known to play a role in maintaining the integrity of the vasculature and thrombotic pathways linked to this, given they surprisingly contain the highest concentration of mHtt of all blood cells.
Methods: We assessed the functional status of platelets by performing ELISA, western blot and RNA sequencing in a cohort of 71 patients and 68 age- and sex-matched healthy control subjects. We further performed haemostasis and platelet depletion tests in the R6/2 HD mouse model.
Results: Our findings indicate that the platelets in HD are dysfunctional with respect to the release of angiogenic factors and functions including thrombosis, angiogenesis and vascular haemostasis.
Conclusion: Taken together, our results provide a better understanding for the impact of mHtt on platelet function.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


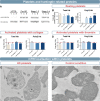

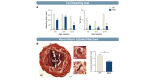
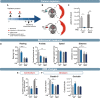
References
-
- Bates GP, Dorsey R, Gusella JF. Huntington disease. Nat Rev Dis Primer 2015;1:nrdp20155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
