Fh15 Blocks the Lipopolysaccharide-Induced Cytokine Storm While Modulating Peritoneal Macrophage Migration and CD38 Expression within Spleen Macrophages in a Mouse Model of Septic Shock
- PMID: 30567900
- PMCID: PMC6300687
- DOI: 10.1128/mSphere.00548-18
Fh15 Blocks the Lipopolysaccharide-Induced Cytokine Storm While Modulating Peritoneal Macrophage Migration and CD38 Expression within Spleen Macrophages in a Mouse Model of Septic Shock
Abstract
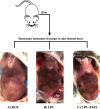
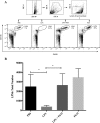
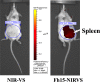
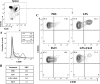
Sepsis caused by Gram-negative bacteria is the consequence of an unrestrained infection that continuously releases lipopolysaccharide (LPS) into the bloodstream, which triggers an uncontrolled systemic inflammatory response leading to multiorgan failure and death. After scrutinizing the immune modulation exerted by a recombinant Fasciola hepatica fatty acid binding protein termed Fh15, our group demonstrated that addition of Fh15 to murine macrophages 1 h prior to LPS stimulation significantly suppresses the expression of proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL1-β). The present study aimed to demonstrate that Fh15 could exert a similar anti-inflammatory effect in vivo using a mouse model of septic shock. Among the novel findings reported in this article, (i) Fh15 suppressed numerous serum proinflammatory cytokines/chemokines when injected intraperitoneally 1 h after exposure of animals to lethal doses of LPS, (ii) concurrently, Fh15 increased the population of large peritoneal macrophages (LPMs) in the peritoneal cavity (PerC) of LPS-injected animals, and (iii) Fh15 downregulated the expression on spleen macrophages of CD38, a cell surface ectoenzyme with a critical role during inflammation. These findings present the first evidence that the recombinant parasitic antigen Fh15 is an excellent modulator of the PerC cell content and in vivo macrophage activation, endorsing Fh15's potential as a drug candidate against sepsis-related inflammatory response.IMPORTANCE Sepsis is a potentially life-threatening complication of an infection. Sepsis is mostly the consequence of systemic bacterial infections leading to exacerbated activation of immune cells by bacterial products, resulting in enhanced release of inflammatory mediators. Lipopolysaccharide (LPS), the major component of the outer membrane of Gram-negative bacteria, is a critical factor in the pathogenesis of sepsis, which is sensed by Toll-like receptor 4 (TLR4). The scientific community highly pursues the development of antagonists capable of blocking the cytokine storm by blocking TLR4. We report here that a recombinant molecule of 14.5 kDa belonging to the Fasciola hepatica fatty acid binding protein (Fh15) is capable of significantly suppressing the LPS-induced cytokine storm in a mouse model of septic shock when administered by the intraperitoneal route 1 h after a lethal LPS injection. These results suggest that Fh15 is an excellent candidate for drug development against endotoxemia.
Keywords: CD38; Fasciola hepatica; cytokines; fatty acid binding protein; macrophages; septic shock.
Copyright © 2018 Ramos-Benitez et al.
Figures


Similar articles
-
Fasciola hepatica GST mu-class suppresses the cytokine storm induced by E. coli-lipopolysaccharide, whereas it modulates the dynamic of peritoneal macrophages in a mouse model and suppresses the classical activation of macrophages.Microbiol Spectr. 2024 Jan 11;12(1):e0347523. doi: 10.1128/spectrum.03475-23. Epub 2023 Nov 29. Microbiol Spectr. 2024. PMID: 38018982 Free PMC article.
-
Recombinant Fasciola hepatica Fatty Acid Binding Protein as a Novel Anti-Inflammatory Biotherapeutic Drug in an Acute Gram-Negative Nonhuman Primate Sepsis Model.Microbiol Spectr. 2021 Dec 22;9(3):e0191021. doi: 10.1128/Spectrum.01910-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937173 Free PMC article.
-
Fasciola hepatica GST mu-class suppresses the cytokine storm induced by E. coli -lipopolysaccharide whereas modulates the dynamic of peritoneal macrophages in a mouse model and suppresses the classical activation of macrophages.bioRxiv [Preprint]. 2023 Aug 10:2023.08.10.552847. doi: 10.1101/2023.08.10.552847. bioRxiv. 2023. Update in: Microbiol Spectr. 2024 Jan 11;12(1):e0347523. doi: 10.1128/spectrum.03475-23. PMID: 37609327 Free PMC article. Updated. Preprint.
-
Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex.Pharmacol Ther. 2003 Nov;100(2):171-94. doi: 10.1016/j.pharmthera.2003.08.003. Pharmacol Ther. 2003. PMID: 14609719 Review.
-
Oral and transdermal administration of lipopolysaccharide safely enhances self-healing ability through the macrophage network.Front Immunol. 2025 Mar 31;16:1563484. doi: 10.3389/fimmu.2025.1563484. eCollection 2025. Front Immunol. 2025. PMID: 40230835 Free PMC article. Review.
Cited by
-
Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2.Nat Prod Bioprospect. 2022 Feb 14;12(1):4. doi: 10.1007/s13659-022-00325-4. Nat Prod Bioprospect. 2022. PMID: 35157175 Free PMC article.
-
Quantitative Proteomics Reveals Fh15 as an Antagonist of TLR4 Downregulating the Activation of NF-κB, Inducible Nitric Oxide, Phagosome Signaling Pathways, and Oxidative Stress of LPS-Stimulated Macrophages.Int J Mol Sci. 2025 Jul 18;26(14):6914. doi: 10.3390/ijms26146914. Int J Mol Sci. 2025. PMID: 40725160 Free PMC article.
-
Fasciola hepatica GST mu-class suppresses the cytokine storm induced by E. coli-lipopolysaccharide, whereas it modulates the dynamic of peritoneal macrophages in a mouse model and suppresses the classical activation of macrophages.Microbiol Spectr. 2024 Jan 11;12(1):e0347523. doi: 10.1128/spectrum.03475-23. Epub 2023 Nov 29. Microbiol Spectr. 2024. PMID: 38018982 Free PMC article.
-
Recombinant Fasciola hepatica Fatty Acid Binding Protein as a Novel Anti-Inflammatory Biotherapeutic Drug in an Acute Gram-Negative Nonhuman Primate Sepsis Model.Microbiol Spectr. 2021 Dec 22;9(3):e0191021. doi: 10.1128/Spectrum.01910-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937173 Free PMC article.
-
Circadian Rhythms in Bacterial Sepsis Pathology: What We Know and What We Should Know.Front Cell Infect Microbiol. 2021 Dec 9;11:773181. doi: 10.3389/fcimb.2021.773181. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34956930 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
