How I treat elderly patients with plasma cell dyscrasias
- PMID: 30568029
- PMCID: PMC6326666
- DOI: 10.18632/aging.101707
How I treat elderly patients with plasma cell dyscrasias
Abstract
Plasma cell dyscrasias are a rare heterogeneous group of hematological disorders which are more prevalent in the older part of the population. The introduction of novel agents, improved understanding of disease biology and better supportive management have improved outcomes considerably and in the era of the aging population the question of how to best manage older patients with plasma cell dyscrasias has never been more relevant. Data on how to treat these patients comes mostly from subgroup analysis as they are underrepresented in clinical trials. This review will cover issues, available evidence and recommendations relevant to diagnosis and management of the older patients with Multiple Myeloma (MM), Waldenstrom Macroglobulinemia (WM) and systemic AL Amyloidosis. What will become increasingly evident is the need to develop and establish the use of disease-specific geriatric assessment (GA) tools. Frailty status assessment using GA tools and moving away from making decisions based merely on chronological age will allow setting clear treatment goals and consequently achieving an optimum balance between effectiveness and toxicity for this complex and heterogeneous group of patients.
Keywords: AL amyloidosis; Multiple Myeloma; Waldenstrom Macroglobulinemia; elderly patients; frailty.
Conflict of interest statement
Figures
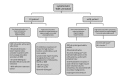

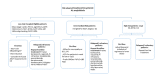
References
-
- United Nations. 2018. http://www.un.org/en/sections/issues-depth/ageing/.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

