Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR
- PMID: 30568035
- PMCID: PMC6338313
- DOI: 10.1172/jci.insight.122695
Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR
Abstract
Background: The ability to restore cystic fibrosis transmembrane regulator (CFTR) function with effective small molecule modulators in patients with cystic fibrosis provides an opportunity to study relationships between CFTR ion channel function, organ level physiology, and clinical outcomes.
Methods: We performed a multisite, prospective, observational study of ivacaftor, prescribed in patients with the G551D-CFTR mutation. Measurements of lung mucociliary clearance (MCC) were performed before and after treatment initiation (1 and 3 months), in parallel with clinical outcome measures.
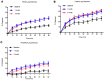
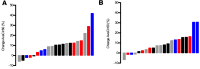
Results: Marked acceleration in whole lung, central lung, and peripheral lung MCC was observed 1 month after beginning ivacaftor and was sustained at 3 months. Improvements in MCC correlated with improvements in forced expiratory volume in the first second (FEV1) but not sweat chloride or symptom scores.
Conclusions: Restoration of CFTR activity with ivacaftor led to significant improvements in MCC. This physiologic assessment provides a means to characterize future CFTR modulator therapies and may help to predict improvements in lung function.
Trial registration: ClinicialTrials.gov, NCT01521338.
Funding: CFF Therapeutics (GOAL11K1).
Keywords: Chloride channels; Diagnostic imaging; Pulmonology.
Conflict of interest statement
Figures