An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment
- PMID: 30568223
- PMCID: PMC6755962
- DOI: 10.1038/s41388-018-0637-x
An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment
Erratum in
-
Correction to: An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment.Oncogene. 2022 Jul;41(27):3584. doi: 10.1038/s41388-022-02356-0. Oncogene. 2022. PMID: 35662285 Free PMC article. No abstract available.
Abstract
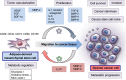
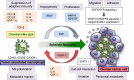
Metastasis is a complex multistep process that involves critical interactions between cancer cells and a variety of stromal components in the tumor microenvironment, which profoundly influence the different aspects of the metastatic cascade and organ tropism of disseminating cancer cells. Ovarian cancer is the most lethal gynecological malignancy and is characterized by peritoneal disseminated metastasis. Evidence has demonstrated that ovarian cancer possesses specific metastatic tropism for the adipose-rich omentum, which has a pivotal role in the creation of the metastatic tumor microenvironment in the intraperitoneal cavity. Considering the distinct biology of ovarian cancer metastasis, the elucidation of the cellular and molecular mechanisms underlying the reciprocal interplay between ovarian cancer cells and surrounding stromal cell types in the adipose-rich metastatic microenvironment will provide further insights into the development of novel therapeutic approaches for patients with advanced ovarian cancer. Herein, we review the biological mechanisms that regulate the highly orchestrated crosstalk between ovarian cancer cells and various cancer-associated stromal cells in the metastatic tumor microenvironment with regard to the omentum by illustrating how different stromal cells concertedly contribute to the development of ovarian cancer metastasis and metastatic tropism for the omentum.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

