STING directly activates autophagy to tune the innate immune response
- PMID: 30568238
- PMCID: PMC6748081
- DOI: 10.1038/s41418-018-0251-z
STING directly activates autophagy to tune the innate immune response
Abstract
STING (stimulator of interferon genes) is a central molecule that binds to cyclic dinucleotides produced by the cyclic GMP-AMP synthase (cGAS) to activate innate immunity against microbial infection. Here we report that STING harbors classic LC-3 interacting regions (LIRs) and mediates autophagy through its direct interaction with LC3. We observed that poly(dA:dT), cGAMP, and HSV-1 induced STING-dependent autophagy and degradation of STING immediately after TBK1 activation. STING induces non-canonical autophagy that is dependent on ATG5, whereas other autophagy regulators such as Beclin1, Atg9a, ULK1, and p62 are dispensable. LIR mutants of STING abolished its interaction with LC3 and its activation of autophagy. Also, mutants that abolish STING dimerization and cGAMP-binding diminished the STING-LC3 interaction and subsequent autophagy, suggesting that STING activation is indispensable for autophagy induction. Our results thus uncover dual functions of STING in activating the immune response and autophagy, and suggest that STING is involved in ensuring a measured innate immune response.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
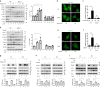

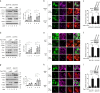


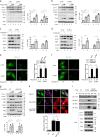

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

