Thermal stress induces glycolytic beige fat formation via a myogenic state
- PMID: 30568302
- PMCID: PMC6328316
- DOI: 10.1038/s41586-018-0801-z
Thermal stress induces glycolytic beige fat formation via a myogenic state
Abstract
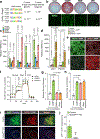
Environmental cues profoundly affect cellular plasticity in multicellular organisms. For instance, exercise promotes a glycolytic-to-oxidative fibre-type switch in skeletal muscle, and cold acclimation induces beige adipocyte biogenesis in adipose tissue. However, the molecular mechanisms by which physiological or pathological cues evoke developmental plasticity remain incompletely understood. Here we report a type of beige adipocyte that has a critical role in chronic cold adaptation in the absence of β-adrenergic receptor signalling. This beige fat is distinct from conventional beige fat with respect to developmental origin and regulation, and displays enhanced glucose oxidation. We therefore refer to it as glycolytic beige fat. Mechanistically, we identify GA-binding protein α as a regulator of glycolytic beige adipocyte differentiation through a myogenic intermediate. Our study reveals a non-canonical adaptive mechanism by which thermal stress induces progenitor cell plasticity and recruits a distinct form of thermogenic cell that is required for energy homeostasis and survival.
Conflict of interest statement
Figures

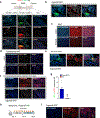

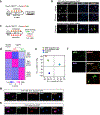

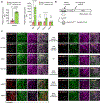

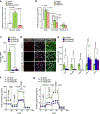

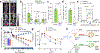
Comment in
-
Fat cells with a sweet tooth.Nature. 2019 Jan;565(7738):167-168. doi: 10.1038/d41586-018-07739-6. Nature. 2019. PMID: 30622347 No abstract available.
-
A new shade of beige.Nat Rev Endocrinol. 2019 Mar;15(3):127. doi: 10.1038/s41574-019-0157-y. Nat Rev Endocrinol. 2019. PMID: 30651635 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

