Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization
- PMID: 30568304
- PMCID: PMC6440214
- DOI: 10.1038/s41586-018-0808-5
Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization
Abstract
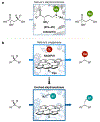
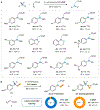
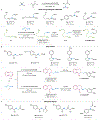
Although abundant in organic molecules, carbon-hydrogen (C-H) bonds are typically considered unreactive and unavailable for chemical manipulation. Recent advances in C-H functionalization technology have begun to transform this logic, while emphasizing the importance of and challenges associated with selective alkylation at a sp3 carbon1,2. Here we describe iron-based catalysts for the enantio-, regio- and chemoselective intermolecular alkylation of sp3 C-H bonds through carbene C-H insertion. The catalysts, derived from a cytochrome P450 enzyme in which the native cysteine axial ligand has been substituted for serine (cytochrome P411), are fully genetically encoded and produced in bacteria, where they can be tuned by directed evolution for activity and selectivity. That these proteins activate iron, the most abundant transition metal, to perform this chemistry provides a desirable alternative to noble-metal catalysts, which have dominated the field of C-H functionalization1,2. The laboratory-evolved enzymes functionalize diverse substrates containing benzylic, allylic or α-amino C-H bonds with high turnover and excellent selectivity. Furthermore, they have enabled the development of concise routes to several natural products. The use of the native iron-haem cofactor of these enzymes to mediate sp3 C-H alkylation suggests that diverse haem proteins could serve as potential catalysts for this abiological transformation, and will facilitate the development of new enzymatic C-H functionalization reactions for applications in chemistry and synthetic biology.
Figures

References
-
- Frey PA & Hegeman AD Enzymatic Reaction Mechanisms (Oxford University Press, New York, 2007), chap. 14.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

