Nanomaterials as photothermal therapeutic agents
- PMID: 30568319
- PMCID: PMC6295417
- DOI: 10.1016/j.pmatsci.2018.07.005
Nanomaterials as photothermal therapeutic agents
Abstract
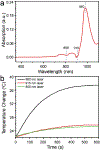
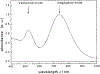
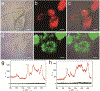
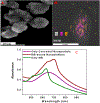
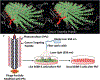
Curing cancer has been one of the greatest conundrums in the modern medical field. To reduce side-effects associated with the traditional cancer therapy such as radiotherapy and chemotherapy, photothermal therapy (PTT) has been recognized as one of the most promising treatments for cancer over recent years. PTT relies on ablation agents such as nanomaterials with a photothermal effect, for converting light into heat. In this way, elevated temperature could kill cancer cells while avoiding significant side effects on normal cells. This theory works because normal cells have a higher heat tolerance than cancer cells. Thus, nanomaterials with photothermal effects have attracted enormous attention due to their selectivity and non-invasive attributes. This review article summarizes the current status of employing nanomaterials with photothermal effects for anti-cancer treatment. Mechanisms of the photothermal effect and various factors affecting photothermal performance will be discussed. Efficient and selective PTT is believed to play an increasingly prominent role in cancer treatment. Moreover, merging PTT with other methods of cancer therapies is also discussed as a future trend.
Keywords: Cancer; Nanomaterials; Photothermal therapy.
Figures
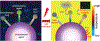

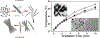
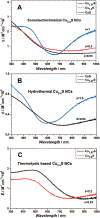
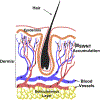
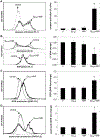
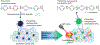



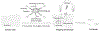
References
-
- Chen Y, Jungsuwadee P, Vore M, Butterfield D, St-Clair D. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interventions 2007;7:2653–63. - PubMed
-
- Edison M, Johns C. Acute and chronic cutaneous reactions to radiotherapy In: Cognetta AB, Mendenhall WM, editors. Radiation therapy for skin cancer. New York: Springer; 2013. p. 55–69.
-
- Matteini P, Tatini F, Cavigli L, Ottaviano S, Ghini G, Pini R. Graphene as a photothermal switch for controlled drug release. Nanoscale 2014;6:7947–53. - PubMed
-
- Tang S, Chen M, Zheng N. Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small 2014;10:3139–44. - PubMed
-
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008;23:217–28. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
