Chemiplasmonics for high-throughput biosensors
- PMID: 30568445
- PMCID: PMC6267718
- DOI: 10.2147/IJN.S186644
Chemiplasmonics for high-throughput biosensors
Abstract
Background: The sensitivity of ELISA for biomarker detection can be significantly increased by integrating fluorescence with plasmonics. In surface-plasmon-coupled emission, the fluorophore emission is generally enhanced through the so-called physical mechanism due to an increase in the local electric field. Despite its fairly high enhancement factors, the use of surface-plasmon-coupled emission for high-throughput and point-of-care applications is still hampered due to the need for expensive focusing optics and spectrometers.
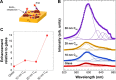
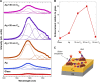
Methods: Here, we describe a new chemiplasmonic-sensing paradigm for enhanced emission through the molecular interactions between aromatic dyes and C60 films on Ag substrates.
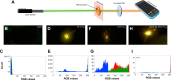
Results: A 20-fold enhancement in the emission from rhodamine B-labeled biomolecules can be readily elicited without quenching its red color emission. As a proof of concept, we demonstrate two model bioassays using: 1) the RhB-streptavidin and biotin complexes in which the dye was excited using an inexpensive laser pointer and the ensuing enhanced emission was recorded by a smartphone camera without the need for focusing optics and 2) high-throughput 96-well plate assay for a model antigen (rabbit immunoglobulin) that showed detection sensitivity as low as 6.6 pM.
Conclusion: Our results show clear evidence that chemiplasmonic sensors can be extended to detect biomarkers in a point-of-care setting through a smartphone in simple normal incidence geometry without the need for focusing optics. Furthermore, chemiplasmonic sensors also facilitate high-throughput screening of biomarkers in the conventional 96-well plate format with 10-20 times higher sensitivity.
Keywords: biosensor; fluorescence; fullerenes; nanosilver; surface plasmons.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Vasan AS, Mahadeo DM, Doraiswami R, Huang Y, Pecht M. Point-of-care biosensor system. Front Biosci. 2013;5(1):39–71. - PubMed
-
- Roda A, Michelini E, Zangheri M, di Fusco M, Calabria D, Simoni P. Smartphone-based biosensors: a critical review and perspectives. TrAC Trends Analyt Chem. 2016;79:317–325.
-
- Hu J, Wang S, Wang L, et al. Advances in paper-based point-of-care diagnostics. Biosens Bioelectron. 2014;54:585–597. - PubMed
-
- World Health Organization World Health Statistics 2014. 2014. [AccessedSeptember 6, 2018]. p. 175. Available from: https://www.who.int/gho/publications/world_health_statistics/2014/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

