A comparison of fulvestrant plus a targeted agent with fulvestrant alone in hormone receptor-positive advanced breast cancer that progressed on prior endocrine therapy: a meta-analysis
- PMID: 30568462
- PMCID: PMC6267349
- DOI: 10.2147/OTT.S166653
A comparison of fulvestrant plus a targeted agent with fulvestrant alone in hormone receptor-positive advanced breast cancer that progressed on prior endocrine therapy: a meta-analysis
Abstract
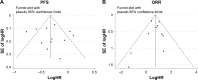
Fulvestrant is recommended for the hormone receptor-positive metastatic breast cancer (MBC) patients progressed during or after prior endocrine therapy. Notably, recent evidence has also demonstrated that adding a targeted agent to fulvestrant conferred a significantly clinical benefit in these patients. Since these results were inconsistent among the studies, this meta-analysis herein was conducted to compare the efficacy and toxicities of the fulvestrant-based combination therapy with fulvestrant monotherapy. Thus, a systemic research was performed in PubMed, Embase, and Cochrane library to identify relevant Phase II or Phase III randomized controlled trials. The progression-free survival (PFS), overall response rate (ORR), and toxicities were evaluated. And HR, risk ratio (RR), and their 95% CIs were employed to complete the pooled analyses. In total, 13 studies with 3,910-hour positive MBC patients progressed on prior endocrine therapy were included in our meta-analysis. Improvements of doublet-agents group were proven in terms of PFS (HR 0.73, 95% CI =0.63-0.86, P=0.000) and ORR (RR 2.07, 95% CI =1.67-2.58, P=0.000). And the further subgroup analysis also demonstrated that fulvestrant in combination with a cyclin-dependent kinase (CDK4/6) inhibitor or a PI3K/mTOR inhibitor was associated with a superior efficacy (RR 2.72, 95% CI =1.93-3.83, P=0.000 and RR 1.60, 95% CI =1.15-2.23, P=0.005, respectively). However, the efficacy was comparable between the other combination strategies and fulvestrant alone. With respect to the adverse effects, adding a targeted agent to fulvestrant also produced more frequent grade 3/4 toxicities (RR 3.86, 95% CI =2.66-5.61, P=0.000). Taken together, combination of fulvestrant with a targeted agent, especially inhibitors targeting CDK4/6 or PI3K/mTOR pathway, may open a new avenue for more effective therapies in relapse or metastatic hormone receptor-positive breast cancer after prior aromatase inhibitors or tamoxifen treatment. In addition, identifying reliable biomarkers to delineate which subgroup of patients will specially benefit from fulvestrant-based combination therapy is warranted.
Keywords: breast cancer; combination therapy; endocrine resistance; fulvestrant.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis.Lancet Oncol. 2020 Feb;21(2):250-260. doi: 10.1016/S1470-2045(19)30804-6. Epub 2019 Dec 16. Lancet Oncol. 2020. PMID: 31859246
-
An evidence-based review of the outcome of fulvestrant plus a targeted agent versus fulvestrant alone in treating hormone receptor-positive endocrine therapy-resistant metastatic breast cancer.Arch Gynecol Obstet. 2019 Nov;300(5):1377-1382. doi: 10.1007/s00404-019-05304-8. Epub 2019 Oct 10. Arch Gynecol Obstet. 2019. PMID: 31599350 Review.
-
Fulvestrant plus targeted agents versus fulvestrant alone for treatment of hormone-receptor positive advanced breast cancer progressed on previous endocrine therapy: a meta-analysis of randomized controlled trials.Breast Cancer. 2017 May;24(3):345-352. doi: 10.1007/s12282-017-0770-3. Epub 2017 Mar 21. Breast Cancer. 2017. PMID: 28324247 Review.
-
CDK4/6 inhibitors, PI3K/mTOR inhibitors, and HDAC inhibitors as second-line treatments for hormone receptor-positive, HER2-negative advanced breast cancer: a network meta-analysis.BMC Cancer. 2023 Aug 29;23(1):805. doi: 10.1186/s12885-023-11290-7. BMC Cancer. 2023. PMID: 37644396 Free PMC article.
-
The efficacy and safety of targeted therapy plus fulvestrant in postmenopausal women with hormone-receptor positive advanced breast cancer: A meta-analysis of randomized-control trials.PLoS One. 2018 Sep 20;13(9):e0204202. doi: 10.1371/journal.pone.0204202. eCollection 2018. PLoS One. 2018. PMID: 30235292 Free PMC article.
Cited by
-
The Value of LncRNA BCAR4 as a Prognostic Biomarker on Clinical Outcomes in Human Cancers.J Cancer. 2019 Oct 15;10(24):5992-6002. doi: 10.7150/jca.35113. eCollection 2019. J Cancer. 2019. PMID: 31762809 Free PMC article.
-
Upregulated expression of LncRNA nicotinamide nucleotide transhydrogenase antisense RNA 1 is correlated with unfavorable clinical outcomes in cancers.BMC Cancer. 2020 Sep 14;20(1):879. doi: 10.1186/s12885-020-07348-5. BMC Cancer. 2020. PMID: 32928135 Free PMC article.
-
Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Oct 1;3(10):e2020312. doi: 10.1001/jamanetworkopen.2020.20312. JAMA Netw Open. 2020. PMID: 33048129 Free PMC article.
-
The prognostic value of long noncoding RNA SNHG16 on clinical outcomes in human cancers: a systematic review and meta-analysis.Cancer Cell Int. 2019 Oct 11;19:261. doi: 10.1186/s12935-019-0971-2. eCollection 2019. Cancer Cell Int. 2019. PMID: 31632195 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Başaran GA, Twelves C, Diéras V, Cortés J, Awada A. Ongoing unmet needs in treating estrogen receptor-positive/HER2-negative metastatic breast cancer. Cancer Treat Rev. 2018;63:144–155. - PubMed
-
- Vidula N, Rugo HS. Emerging data on improving response to hormone therapy: the role of novel targeted agents. Expert Rev Anticancer Ther. 2018;18(1):3–18. - PubMed
-
- De Placido S, Pronzato P. Treatment options in HR+/HER2− advanced breast cancer patients pretreated with nonsteroidal aromatase inhibitors: what does current evidence tell us? Future Oncol. 2015;11(6):975–981. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

