Treatment persistence of subcutaneous TNF inhibitors among Australian patients with immune-mediated rheumatic disease (IMRD)
- PMID: 30568519
- PMCID: PMC6267492
- DOI: 10.2147/OARRR.S179704
Treatment persistence of subcutaneous TNF inhibitors among Australian patients with immune-mediated rheumatic disease (IMRD)
Abstract
Introduction: To describe the persistence of treatment with subcutaneous tumor necrosis factor inhibitors (TNFi) adalimumab, etanercept, and golimumab in immune-mediated rheumatic disease (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis) by treatment sequence (first-line treatment, second-line or further lines of treatment).
Methods: A retrospective cohort analysis was conducted using the Australian Commonwealth Department of Human Services Pharmaceutical Benefits Scheme 10% sample data from January 1, 2010, to June 30, 2016. Pharmaceutical Benefits Scheme indications were used to identify patient prescriptions for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. A patient was considered persistent until a 3-month gap period where a prescription was not dispensed. The 3-month gap interval was chosen because only 1% of all discontinuations occurred beyond this 3-month period.
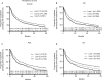
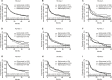
Results: Data from 2,612 first-line patients were included. Treatment discontinuation among first-line patients treated with etanercept or adalimumab was not significantly different from those treated with golimumab (HR 1.10, 95% CI 0.95-1.28, P=0.22; HR 1.06, 95% CI 0.93-1.22, P=0.39; respectively). Among the 1,276 patients in the second-line cohort (etanercept=41%, adalimumab=41%, golimumab=18%) discontinuation was significantly higher for patients on etanercept compared with golimumab (HR 1.24, 95% CI 1.03-1.50, P=0.03); but not for adalimumab compared with golimumab (HR 1.11, 95% CI 0.91-1.34, P=0.31). In the third-line setting, treatment persistence with etanercept was longer than golimumab (HR 0.75, 95% CI 0.59-0.96, P=0.02), but there was no difference between golimumab and adalimumab. Similar findings occurred in the propensity score matched population.
Conclusion: Our study shows there is variance in real-world persistence to TNFi in patients with immune-mediated rheumatic disease by line of therapy, with the time on therapy decreasing by line. Australian persistence has been reported at lower overall rates than international evidence.
Keywords: ankylosing spondylitis; arthritis; psoriatic; rheumatoid arthritis; treatment persistence; tumor necrosis factor inhibitors.
Conflict of interest statement
Disclosure MA and MH are employees of Janssen-Cilag Pty Ltd., who market golimumab (SIMPONI). PJ is an employee of Prospection Pty Ltd. who were contracted by Janssen-Cilag Pty Ltd. to perform the analyses. The authors report no other conflicts of interest in this work.
Figures


References
-
- Favalli EG, Raimondo MG, Becciolini A, Crotti C, Biggioggero M, Caporali R. The management of first-line biologic therapy failures in rheumatoid arthritis: Current practice and future perspectives. Autoimmun Rev. 2017;16(12):1185–1195. - PubMed
-
- Koncz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther. 2010;10(9):1367–1378. - PubMed
-
- Dalén J, Svedbom A, Black CM, et al. Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int. 2016;36(7):987–995. - PubMed
-
- Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C. Utilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther. 2014;36(5):737–747. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

