Quantitation of ERK1/2 inhibitor cellular target occupancies with a reversible slow off-rate probe
- PMID: 30568786
- PMCID: PMC6253716
- DOI: 10.1039/c8sc02754d
Quantitation of ERK1/2 inhibitor cellular target occupancies with a reversible slow off-rate probe
Abstract
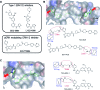
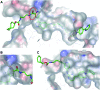
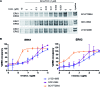
Target engagement is a key concept in drug discovery and its direct measurement can provide a quantitative understanding of drug efficacy and/or toxicity. Failure to demonstrate target occupancy in relevant cells and tissues has been recognised as a contributing factor to the low success rate of clinical drug development. Several techniques are emerging to quantify target engagement in cells; however, in situ measurements remain challenging, mainly due to technical limitations. Here, we report the development of a non-covalent clickable probe, based on SCH772984, a slow off-rate ERK1/2 inhibitor, which enabled efficient pull down of ERK1/2 protein via click reaction with tetrazine tagged agarose beads. This was used in a competition setting to measure relative target occupancy by selected ERK1/2 inhibitors. As a reference we used the cellular thermal shift assay, a label-free biophysical assay relying solely on ligand-induced thermodynamic stabilization of proteins. To validate the EC50 values measured by both methods, the results were compared with IC50 data for the phosphorylation of RSK, a downstream substrate of ERK1/2 used as a functional biomarker of ERK1/2 inhibition. We showed that a slow off-rate reversible probe can be used to efficiently pull down cellular proteins, significantly extending the potential of the approach beyond the need for covalent or photoaffinity warheads.
Figures

References
-
- Morgan P., Van Der Graaf P. H., Arrowsmith J., Feltner D. E., Drummond K. S., Wegner C. D., Street S. D. A. Drug Discovery Today. 2012;17:419–424. - PubMed
-
- Cook D., Brown D., Alexander R., March R., Morgan P., Satterthwaite G., Pangalos M. N. Nat. Rev. Drug Discovery. 2014;13:419–431. - PubMed
-
- Bunnage M. E., Chekler E. L. P., Jones L. H. Nat. Chem. Biol. 2013;9:195–199. - PubMed
-
- Schürmann M., Janning P., Ziegler S., Waldmann H. Cell Chem. Biol. 2016;23:435–441. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

