Adalimumab and Infliximab in Crohn's disease - real life data from a national retrospective cohort study
- PMID: 30568821
- PMCID: PMC6256158
- DOI: 10.12865/CHSJ.42.02.01
Adalimumab and Infliximab in Crohn's disease - real life data from a national retrospective cohort study
Abstract
Aim: to compare the efficacy and safety of Adalimumab(ADA) and Infliximab(IFX), in a large Romanian population and to identify predictors of response. Methods We performed a national retrospective cohort study including 265 patients (136 ADA, 129 IFX) between 2008-2014. Binary logistic regression was performed with the statistical program Minitab.
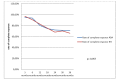
Results: Patients were half women, with a median age of 36, a median disease duration of 2.5 years, 80% received Azathioprine. Mean therapy duration was 20 months in ADA group and 36 months in IFX group. Complete response to Adalimumab respectively Infliximab was recorded in 77%vs.65%, secondary loss of response in 18%vs.28%, statistically comparable. We failed to identify predictors of response. In 79.2%of patients with secondary loss of response to ADA, the dose was escalated, 12.5% were switched to Infliximab. In 70%of patients that lost response to IFX, the dose was increased, 30% were switched to Adalimumab.
Conclusions: Adalimumab and Infliximab have similar efficacy, with a complete response rate of~70%. In case of secondary loss of response to IFX, the best solution is to switch to ADA, with 83% response rate, while in case of secondary loss of response to ADA, increasing the dose leads to 84 % response rate.
Keywords: Adalimumab; Crohn's disease; Infliximab.
Figures
References
-
- Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. for the European Crohn's and Colitis Organisation (ECCO). The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. Journal of Crohn's and Colitis. 2010;4:7–27. - PubMed
-
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, L�fberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–530. - PubMed
-
- D'Haens G, Baert F, Van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660–667. - PubMed
-
- Dignass A, Van Assche G, Lindsay JO, L�mann M, S�derholm J, Colombel JF, et al. for the European Crohn's and Colitis Organisation (ECCO). The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Current management. Journal of Crohn's and Colitis. 2010;4:28–62. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials