MicroRNA‑206 exerts anti‑oncogenic functions in esophageal squamous cell carcinoma by suppressing the c‑Met/AKT/mTOR pathway
- PMID: 30569129
- PMCID: PMC6390054
- DOI: 10.3892/mmr.2018.9775
MicroRNA‑206 exerts anti‑oncogenic functions in esophageal squamous cell carcinoma by suppressing the c‑Met/AKT/mTOR pathway
Abstract
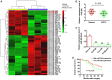
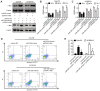
Increasing evidence suggests that the dysregulation of microRNAs (miRNAs) has an important role in the progression of human cancer, including ESCC. However, the exact functions and mechanisms of miRNAs in ESCC remain largely unclear. The aim of the present study was to investigate the expression and biological functions of miRNAs in ESCC and reveal the underlying molecular mechanisms. miRNA microarray and reverse transcription‑quantitative polymerase chain reaction analyses were performed, which identified and confirmed that miR‑206 was significantly downregulated in ESCC tissues and cell lines. Its low expression was associated with lymph node metastasis, advanced TNM stage and N classification, as well as poorer overall survival in patients with ESCC. CCK‑8 and flow cytometry assays demonstrated that ectopic miR‑206 expression inhibited ESCC cell proliferation and induced cell apoptosis. In addition, MET proto‑oncogene, receptor tyrosine kinase (c‑Met), a well‑known oncogene, was a direct target of miR‑206. An inverse correlation between the levels of miR‑206 and c‑Met mRNA in ESCC tissue samples was confirmed. Notably, c‑Met overexpression inhibited the effects of miR‑206 on the proliferation and apoptosis of ESCC cells. Additionally, it was confirmed that the tumor‑suppressive functions of miR‑206 may have contributed to the inactivation of the c‑Met/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling pathway. In conclusion, the findings of the present study suggested that miR‑206 exerts its anti‑cancer functions via the c‑Met/AKT/mTOR signaling pathway, providing a novel candidate prognostic factor and a potential therapeutic target in ESCC.
Keywords: esophageal squamous cell carcinoma; microRNA‑206; c-Met; AKT/mTOR pathway.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

