Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer
- PMID: 30569186
- PMCID: PMC6451716
- DOI: 10.1007/s00259-018-4236-4
Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer
Abstract
Purpose: The first aim of this study was to evaluate 68Ga-PSMAHBED-CC conjugate 11 positron emission tomography (PSMA PET) parameters for assessment of response to 177Lu-PSMA-617 radioligand therapy (RLT) in patients with metastatic castration-resistant prostate cancer (mCRPC). The second aim was to investigate factors associated with overall survival (OS).
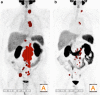
Methods: We retrospectively assessed mean standardized uptake values (SUVmean) and total tumor volumes (TTV) on PSMA PET in 38 of 55 mCRPC patients before and after RLT. PSA testing and PSMA PET/CT(MRI) imaging were performed during the 8 weeks before and the 6 weeks after RLT. PSMA PET and CT(MRI) images were reviewed separately according to the modified PET Response Criteria in Solid Tumors (mPERCIST) and RECIST1.1. The results were compared with PSA responses. Associations between OS and the RECIST evaluation and changes in SUVmean, TTV, and PSA, CRP, LDH, hemoglobin and ALP levels were determined in a univariable survival analysis.
Results: The median PSA level at the time of pretherapy PSMA PET/CT(MRI) was 60.8 ng/ml (IQR 15.4, 264.2 ng/ml). After RLT the median PSA level decreased by 44%, TTV by 45.1%, SUVmean by 25.8% and RECIST by 11.3%. A PSA response was seen in 18 patients (47.4%), stable disease in 12 (31.6%) and progressive disease in 8 (21.1%). Contrary to the changes in SUVmean and the RECIST evaluation, the change in TTV was significantly associated with PSA response (p = 0.15, p = 0.58, and p < 0.001, respectively). After a median follow-up of 17 months (IQR 8.0, 24.2 months), 11 patients (28.9%) had died of their prostate cancer. The changes in both TTV and PSA levels were associated with OS (HR 1.001, 95% CI 1-1.003, p = 0.04, and HR 1.004, 95% CI 1.001-1.008, p = 0.01, respectively), while the changes in SUVmean and the RECIST evaluation were not. The pre-therapy CRP level was also associated with OS (HR 1.07, 95% CI 1.009-1.14, p = 0.02).
Conclusion: TTV on PSMA PET seems to be a reliable parameter for response assessment in mCRPC patients undergoing RLT and might overcome the limitations of RECIST in prostate cancer. Furthermore, the change in TTV was significantly associated with OS in our cohort.
Keywords: Hybrid imaging; Metastatic prostate cancer; PET/CT; PET/MRI; PSMA ligand.
Conflict of interest statement
Disclosure statements
The authors have nothing to disclose.
Conflicts of interest
None.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


References
-
- Bahl A, Oudard S, Tombal B, Özgüroĝlu M, Hansen S, Kocak I, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24:2402–2408. doi: 10.1093/annonc/mdt194. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

