Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis
- PMID: 30569273
- PMCID: PMC6402971
- DOI: 10.1007/s00125-018-4786-9
Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis
Abstract
Aims/hypothesis: The long-term effects of successful immune therapies for treatment of type 1 diabetes have not been well studied. The Autoimmunity-Blocking Antibody for Tolerance (AbATE) trial evaluated teplizumab, an Fc receptor non-binding humanised anti-CD3 monoclonal antibody in individuals with new-onset type 1 diabetes, and ended in 2011. Clinical drug-treated responders showed an increased frequency of 'partially exhausted' CD8+ T cells. We studied the clinical, immunological and metabolic status of participants after an average follow-up of 7 years.
Methods: Participants with detectable C-peptide at year 2 of AbATE returned for follow-up. C-peptide responses were assessed by 4 h mixed-meal tolerance test. Autoantibodies and HbA1c levels were measured and average daily insulin use was obtained from patient logs. Peripheral blood mononuclear cells were analysed by flow cytometry and cytokine release.
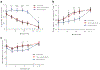
Results: Fifty-six per cent of the original participants returned. Three of the original control group who did not return had lost all detectable C-peptide by the end of the 2 year trial. The C-peptide responses to a mixed-meal tolerance test were similar overall in the drug vs control group of participants but were significantly improved, with less loss of C-peptide, in drug-treated responders identified at 1 year. However, the improvements in C-peptide response were not associated with lower HbA1c levels or insulin use. Drug-treated responders showed a significantly increased frequency of programmed cell death protein 1-positive central memory and anergic CD8+ T cells at follow-up.
Conclusions/interpretation: These findings suggest there is reduced decline in C-peptide and persistent immunological responses up to 7 years after diagnosis of diabetes in individuals who respond to teplizumab.
Trial registration: ClinicalTrials.gov NCT02067923; the protocol is available at www.immunetolerance.org (ITN027AI).
Keywords: Anergy; Anti-CD3; C-peptide; Exhaustion; T cells; Teplizumab; Type 1 diabetes.
Figures

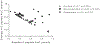
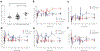
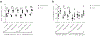

Comment in
-
A future for CD3 antibodies in immunotherapy of type 1 diabetes.Diabetologia. 2019 Apr;62(4):578-581. doi: 10.1007/s00125-018-4808-7. Epub 2019 Jan 5. Diabetologia. 2019. PMID: 30612137 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

