Neurological update: MOG antibody disease
- PMID: 30569382
- PMCID: PMC6469662
- DOI: 10.1007/s00415-018-9122-2
Neurological update: MOG antibody disease
Abstract
Myelin oligodendrocyte glycoprotein (MOG) antibody disease (MOG-AD) is now recognised as a nosological entity with specific clinical and paraclinical features to aid early diagnosis. Although no age group is exempt, median age of onset is within the fourth decade of life, with optic neuritis being the most frequent presenting phenotype. Disease course can be either monophasic or relapsing, with subsequent relapses most commonly involving the optic nerve. Residual disability develops in 50-80% of patients, with transverse myelitis at onset being the most significant predictor of long-term outcome. Recent advances in MOG antibody testing offer improved sensitivity and specificity. To avoid misdiagnosis, MOG antibody testing should be undertaken in selected cases presenting clinical and paraclinical features that are felt to be in keeping with MOG-AD, using a validated cell-based assay. MRI characteristics can help in differentiating MOG-AD from other neuroinflammatory disorders, including multiple sclerosis and neuromyelitis optica. Cerebrospinal fluid oligoclonal bands are uncommon. Randomised control trials are limited, but observational open-label experience suggests a role for high-dose steroids and plasma exchange in the treatment of acute attacks, and for immunosuppressive therapies, such as steroids, oral immunosuppressants and rituximab as maintenance treatment.
Keywords: Cerebrospinal fluid (CSF); MRI; Multiple sclerosis (MS); Myelin oligodendrocyte glycoprotein (MOG) antibodies; Neuromyelitis optica (NMO); Optic neuritis (ON).
Conflict of interest statement
Dr. R. Wynford-Thomas’ clinical research fellow Grant is co-funded by Novartis. Dr. A. Jacob has received research Grants from Biogen Idec, Alexion Pharmaceuticals and speaker fees from Biogen, Chugai, Sanofi-Genzyme and Terumo-BC. Dr. V. Tomassini has received research support and honoraria from Biogen Idec, and honoraria and travel Grants from Biogen Idec and Novartis.
Figures
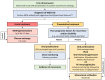
References
-
- Schluesener H, et al. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987;139(12):4016–4021. - PubMed
-
- Linington C, Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG) J Neuroimmunol. 1987;17(1):61–69. doi: 10.1016/0165-5728(87)90031-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

