Regioselective Manipulation of GlcNAc Provides Allosamine, Lividosamine, and Related Compounds
- PMID: 30569712
- PMCID: PMC6343366
- DOI: 10.1021/acs.joc.8b01949
Regioselective Manipulation of GlcNAc Provides Allosamine, Lividosamine, and Related Compounds
Abstract
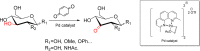
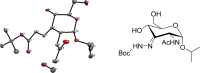
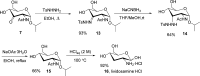
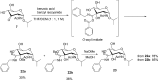
Palladium-catalyzed oxidation of isopropyl N-acetyl-α-d-glucosamine (GlcNAc) is used to prepare the rare sugars allosamine, lividosamine, and related compounds with unprecedented selectivity. The Passerini reaction applied on 3-keto-GlcNAc provides an entry into branching of the carbon skeleton in this compound.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Lenagh-Snow G. M. J.; Araújo N.; Jenkinson S. F.; Martínez R. F.; Shimada Y.; Yu C.-Y.; Kato A.; Fleet G. W. J. Azetidine Iminosugars from the Cyclization of 3,5-Di-O-triflates of α-Furanosides and of 2,4-Di-O-triflates of β-Pyranosides Derived from Glucose. Org. Lett. 2012, 14, 2142–2145. 10.1021/ol300669v. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

