Redox Regulation of Ion Channels and Receptors in Pulmonary Hypertension
- PMID: 30569735
- PMCID: PMC7061297
- DOI: 10.1089/ars.2018.7699
Redox Regulation of Ion Channels and Receptors in Pulmonary Hypertension
Abstract
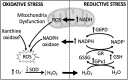
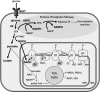
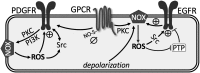
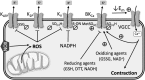
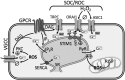
Significance: Pulmonary hypertension (PH) is characterized by elevated vascular resistance due to vasoconstriction and remodeling of the normally low-pressure pulmonary vasculature. Redox stress contributes to the pathophysiology of this disease by altering the regulation and activity of membrane receptors, K+ channels, and intracellular Ca2+ homeostasis. Recent Advances: Antioxidant therapies have had limited success in treating PH, leading to a growing appreciation that reductive stress, in addition to oxidative stress, plays a role in metabolic and cell signaling dysfunction in pulmonary vascular cells. Reactive oxygen species generation from mitochondria and NADPH oxidases has substantial effects on K+ conductance and membrane potential, and both receptor-operated and store-operated Ca2+ entry. Critical Issues: Some specific redox changes resulting from oxidation, S-nitrosylation, and S-glutathionylation are known to modulate membrane receptor and ion channel activity in PH. However, many sites of regulation that have been elucidated in nonpulmonary cell types have not been tested in the pulmonary vasculature, and context-specific molecular mechanisms are lacking. Future Directions: Here, we review what is known about redox regulation of membrane receptors and ion channels in PH. Further investigation of the mechanisms involved is needed to better understand the etiology of PH and develop better targeted treatment strategies.
Keywords: NADPH oxidase; calcium homeostasis; hypoxia; potassium channels; reactive oxygen species; reductive stress.
Figures

References
-
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, and Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004 - PubMed
-
- Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, Hammoudi N, Kho C, Lee A, Ibáñez B, García-Alvarez A, Zsebo K, Maron BA, Plataki M, Fuster V, Leopold JA, and Hajjar RJ. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J Am Coll Cardiol 67: 2032–2046, 2016 - PMC - PubMed
-
- Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, and Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 52: 751–762, 2018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

