Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease
- PMID: 30570675
- PMCID: PMC6426988
- DOI: 10.1007/s00401-018-1947-3
Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease
Abstract
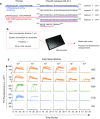
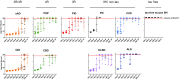
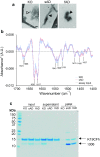
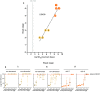
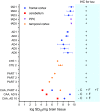
Alzheimer disease (AD) and chronic traumatic encephalopathy (CTE) involve the abnormal accumulation in the brain of filaments composed of both three-repeat (3R) and four-repeat (4R) (3R/4R) tau isoforms. To probe the molecular basis for AD's tau filament propagation and to improve detection of tau aggregates as potential biomarkers, we have exploited the seeded polymerization growth mechanism of tau filaments to develop a highly selective and ultrasensitive cell-free tau seed amplification assay optimized for AD (AD real-time quaking-induced conversion or AD RT-QuIC). The reaction is based on the ability of AD tau aggregates to seed the formation of amyloid fibrils made of certain recombinant tau fragments. AD RT-QuIC detected seeding activity in AD (n = 16) brains at dilutions as extreme as 107-1010-fold, but was 102-106-fold less responsive when seeded with brain from most cases of other types of tauopathy with comparable loads of predominant 3R or 4R tau aggregates. For example, AD brains had average seeding activities that were orders of magnitude higher than Pick disease brains with predominant 3R tau deposits, but the opposite was true using our previously described Pick-optimized tau RT-QuIC assay. CTE brains (n = 2) had seed concentrations comparable to the weakest of the AD specimens, and higher than 3 of 4 specimens with 3R/4R primary age-related tauopathy. AD seeds shared properties with the tau filaments found in AD brains, as AD seeds were sarkosyl-insoluble, protease resistant, and reactive with tau antibodies. Moreover, AD RT-QuIC detected as little as 16 fg of pure synthetic tau fibrils. The distinctive seeding activity exhibited by AD and CTE tau filaments compared to other types of tauopathies in these seeded polymerization reactions provides a mechanistic basis for their consistent propagation as specific conformers in patients with 3R/4R tau diseases. Importantly, AD RT-QuIC also provides rapid ultrasensitive quantitation of 3R/4R tau-seeding activity as a biomarker.
Keywords: Alzheimer disease; Biomarker; Chronic traumatic encephalopathy; Diagnosis; RT-QuIC; Seeds; Tau aggregate; Tauopathy.
Conflict of interest statement
Conflict of interest
AK, ES, MM, and BC are named inventors on a PCT patent application (PCT/US2017/069024) related to the technology described herein. The other authors declare that they have no other competing interests.
Ethics approval
None required; all samples analyzed were from deceased, de-identified individuals. Nonetheless, exemption #13437 from Office of Human Subjects Research was obtained by BC.
Consent for publication
Not applicable
Availability of data and material
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Figures


References
-
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG005131/AG/NIA NIH HHS/United States
- R21 NS105498/NS/NINDS NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- R01 NS069566/NS/NINDS NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- R01 NS076896/NS/NINDS NIH HHS/United States
- P30 AG072973/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- ZIA AI001086/ImNIH/Intramural NIH HHS/United States
- P30 AG19610/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R21 NS110409/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

