Malaria, anemia, and invasive bacterial disease: A neutrophil problem?
- PMID: 30570786
- PMCID: PMC6487965
- DOI: 10.1002/JLB.3RI1018-400R
Malaria, anemia, and invasive bacterial disease: A neutrophil problem?
Abstract
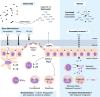
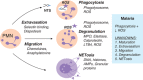
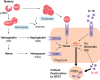
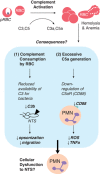
Invasive bacterial disease is well described in immunocompromised hosts, including those with malaria infection. One bacterial infection frequently observed in children with Plasmodium falciparum infection is nontyphoidal salmonella (NTS) infection, in which a typically intestinal infection becomes systemic with serious, often fatal, consequences. In this review, we consider the role of malaria-induced immunoregulatory responses in tipping the balance from tissue homeostasis during malaria infection to risk of invasive NTS. Also, neutrophils are crucial in the clearance of NTS but their ability to mount an oxidative burst and kill intracellular Salmonella is severely compromised during, and for some time after, an acute malaria infection. Here, we summarize the evidence linking malaria and invasive NTS infections; describe the role of neutrophils in clearing NTS infections; review evidence for neutrophil dysfunction in malaria infections; and explore roles of heme oxygenase-1, IL-10, and complement in mediating this dysfunction. Finally, given the epidemiological evidence that low density, subclinical malaria infections pose a risk for invasive NTS infections, we consider whether the high prevalence of such infections might underlie the very high incidence of invasive bacterial disease across much of sub-Saharan Africa.
Keywords: IL-10; anemia; heme oxygenase-1; malaria; neutrophil; salmonella; sepsis.
©2018 Society for Leukocyte Biology.
Figures
References
-
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. - PubMed
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. - PubMed
-
- Antonelli M. Sepsis and septic shock: pro‐inflammatory or anti‐inflammatory state. J Chemother. 1999;11:536–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

