Voluntary wheel running prevents salt-induced endothelial dysfunction: role of oxidative stress
- PMID: 30571282
- PMCID: PMC6397416
- DOI: 10.1152/japplphysiol.00421.2018
Voluntary wheel running prevents salt-induced endothelial dysfunction: role of oxidative stress
Abstract
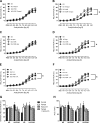
Diets high in salt can lead to endothelial dysfunction, a nontraditional risk factor for cardiovascular disease (CVD). Exercise is known to reduce CVD risk; however, it remains unknown whether chronic physical activity can attenuate salt-induced endothelial dysfunction independent of blood pressure (BP) and whether these changes are due to an upregulation in endogenous antioxidants. Eight-week-old Sprague-Dawley rats were fed either a normal (NS; 0.49%)- or a high (HS; 4.0%)-salt diet and further divided into voluntary wheel running (NS-VWR, HS-VWR) and sedentary (NS, HS) groups for 6 wk. BP was measured weekly and remained unchanged within groups ( P = 0.373). Endothelium-dependent relaxation (EDR) was impaired in the femoral artery of HS compared with NS (38.6 ± 4.0% vs. 65.0 ± 3.6%; P = 0.013) animals, whereas it was not different between NS and HS-VWR (73.4 ± 6.4%; P = 0.273) animals. Incubation with the antioxidants TEMPOL ( P = 0.024) and apocynin ( P = 0.013) improved EDR in HS animals, indicating a role for reactive oxygen species (ROS). Wheel running upregulated the antioxidant superoxide dismutase-2 (SOD-2) ( P = 0.011) under HS conditions and lowered NOX4 and Gp91-phox, two subunits of NADPH oxidase. Wheel running elevated phosphorylated endothelial nitric oxide synthase (eNOS) ( P = 0.014) in HS-fed rats, demonstrating a role for physical activity and eNOS activity under HS conditions. Finally, there was a reduction in EDR ( P = 0.038) when femoral arteries from NS-VWR animals were incubated with TEMPOL or apocynin, suggesting there may be a critical level of ROS needed to maintain endothelial function. In summary, physical activity protected HS-fed rats from reductions in endothelial function, likely through increased SOD-2 levels and reduced oxidative stress. NEW & NOTEWORTHY Our data suggest that voluntary wheel running can prevent impairments in endothelium-dependent relaxation in the femoral artery of rats fed a high-salt diet. This appears to be independent of blood pressure and mediated through a decrease in expression of NADPH oxidases as a result of physical activity. These data suggest that increased chronic physical activity can protect the vasculature from a diet high in salt, likely through a reduction in oxidative stress.
Keywords: endothelium; exercise; sodium.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Similar articles
-
Voluntary Wheel Running Attenuates Salt-Induced Vascular Stiffness Independent of Blood Pressure.Am J Hypertens. 2019 Nov 15;32(12):1162-1169. doi: 10.1093/ajh/hpz128. Am J Hypertens. 2019. PMID: 31401651 Free PMC article.
-
Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension.Am J Physiol Heart Circ Physiol. 2009 Mar;296(3):H833-9. doi: 10.1152/ajpheart.01096.2008. Epub 2009 Jan 16. Am J Physiol Heart Circ Physiol. 2009. PMID: 19151253
-
Nicorandil prevents endothelial dysfunction due to antioxidative effects via normalisation of NADPH oxidase and nitric oxide synthase in streptozotocin diabetic rats.Cardiovasc Diabetol. 2011 Nov 23;10:105. doi: 10.1186/1475-2840-10-105. Cardiovasc Diabetol. 2011. PMID: 22107602 Free PMC article.
-
Nitric oxide and oxidative stress in vascular disease.Pflugers Arch. 2010 May;459(6):923-39. doi: 10.1007/s00424-010-0808-2. Epub 2010 Mar 21. Pflugers Arch. 2010. PMID: 20306272 Review.
-
Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function.Oxid Med Cell Longev. 2020 Sep 26;2020:1496462. doi: 10.1155/2020/1496462. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33062134 Free PMC article. Review.
Cited by
-
Salty Subjects: Unpacking Racial Differences in Salt-Sensitive Hypertension.Curr Hypertens Rep. 2024 Jan;26(1):43-58. doi: 10.1007/s11906-023-01275-z. Epub 2023 Oct 25. Curr Hypertens Rep. 2024. PMID: 37878224 Free PMC article. Review.
-
Getting to the Heart of the Matter: Exploring the Intersection of Cardiovascular Disease, Sex and Race and How Exercise, and Gut Microbiota Influence these Relationships.Rev Cardiovasc Med. 2025 Feb 20;26(2):26430. doi: 10.31083/RCM26430. eCollection 2025 Feb. Rev Cardiovasc Med. 2025. PMID: 40026503 Free PMC article. Review.
-
Exercise-induced cardiac mitochondrial reorganization and enhancement in spontaneously hypertensive rats.Pflugers Arch. 2024 Jul;476(7):1109-1123. doi: 10.1007/s00424-024-02956-7. Epub 2024 Apr 16. Pflugers Arch. 2024. PMID: 38625371
-
The Interplay between Cardiovascular Disease, Exercise, and the Gut Microbiome.Rev Cardiovasc Med. 2022 Oct 27;23(11):365. doi: 10.31083/j.rcm2311365. eCollection 2022 Nov. Rev Cardiovasc Med. 2022. PMID: 39076202 Free PMC article. Review.
-
Voluntary Wheel Running Attenuates Salt-Induced Vascular Stiffness Independent of Blood Pressure.Am J Hypertens. 2019 Nov 15;32(12):1162-1169. doi: 10.1093/ajh/hpz128. Am J Hypertens. 2019. PMID: 31401651 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

