Muscle contracture and passive mechanics in cerebral palsy
- PMID: 30571285
- PMCID: PMC6589815
- DOI: 10.1152/japplphysiol.00278.2018
Muscle contracture and passive mechanics in cerebral palsy
Abstract
Skeletal muscle contractures represent the permanent shortening of a muscle-tendon unit, resulting in loss of elasticity and, in extreme cases, joint deformation. They may result from cerebral palsy, spinal cord injury, stroke, muscular dystrophy, and other neuromuscular disorders. Contractures are the prototypic and most severe clinical presentation of increased passive mechanical muscle force in humans, often requiring surgical correction. Intraoperative experiments demonstrate that high muscle passive force is associated with sarcomeres that are abnormally stretched, although otherwise normal, with fewer sarcomeres in series. Furthermore, changes in the amount and arrangement of collagen in the extracellular matrix also increase muscle stiffness. Structural light and electron microscopy studies demonstrate that large bundles of collagen, referred to as perimysial cables, may be responsible for this increased stiffness and are regulated by interaction of a number of cell types within the extracellular matrix. Loss of muscle satellite cells may be related to changes in both sarcomeres and extracellular matrix. Future studies are required to determine the underlying mechanism for changes in muscle satellite cells and their relationship (if any) to contracture. A more complete understanding of this mechanism may lead to effective nonsurgical treatments to relieve and even prevent muscle contractures.
Keywords: biomechanics; cerebral palsy; extracellular matrix; sarcomere length; skeletal muscle mechanics.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
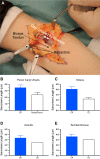
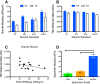
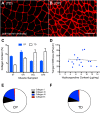

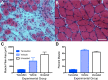
Similar articles
-
Does a Reduced Number of Muscle Stem Cells Impair the Addition of Sarcomeres and Recovery from a Skeletal Muscle Contracture? A Transgenic Mouse Model.Clin Orthop Relat Res. 2020 Apr;478(4):886-899. doi: 10.1097/CORR.0000000000001134. Clin Orthop Relat Res. 2020. PMID: 32011372 Free PMC article.
-
Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length.J Physiol. 2011 May 15;589(Pt 10):2625-39. doi: 10.1113/jphysiol.2010.203364. Epub 2011 Mar 21. J Physiol. 2011. PMID: 21486759 Free PMC article.
-
Pathophysiology of muscle contractures in cerebral palsy.Phys Med Rehabil Clin N Am. 2015 Feb;26(1):57-67. doi: 10.1016/j.pmr.2014.09.005. Phys Med Rehabil Clin N Am. 2015. PMID: 25479779 Free PMC article. Review.
-
Collagen architecture and biomechanics of gracilis and adductor longus muscles from children with cerebral palsy.J Physiol. 2024 Jul;602(14):3489-3504. doi: 10.1113/JP285988. Epub 2024 Jun 20. J Physiol. 2024. PMID: 39008710 Free PMC article.
-
Are mechanically sensitive regulators involved in the function and (patho)physiology of cerebral palsy-related contractures?J Muscle Res Cell Motil. 2017 Aug;38(3-4):317-330. doi: 10.1007/s10974-017-9489-1. Epub 2017 Nov 30. J Muscle Res Cell Motil. 2017. PMID: 29190010 Review.
Cited by
-
Clinical Relevance of State-of-the-Art Analysis of Surface Electromyography in Cerebral Palsy.Front Neurol. 2020 Dec 11;11:583296. doi: 10.3389/fneur.2020.583296. eCollection 2020. Front Neurol. 2020. PMID: 33362693 Free PMC article. Review.
-
Muscle physiology and the science of tone agents.Pediatr Res. 2025 Feb 10. doi: 10.1038/s41390-025-03918-0. Online ahead of print. Pediatr Res. 2025. PMID: 39930248 Review.
-
The effects of eccentric exercise on passive hamstring muscle stiffness: Comparison of shear-wave elastography and passive knee torque outcomes.Eur J Transl Myol. 2022 Jun 6;32(2):10567. doi: 10.4081/ejtm.2022.10567. Eur J Transl Myol. 2022. PMID: 35666465 Free PMC article.
-
The Energy of Muscle Contraction. I. Tissue Force and Deformation During Fixed-End Contractions.Front Physiol. 2020 Aug 31;11:813. doi: 10.3389/fphys.2020.00813. eCollection 2020. Front Physiol. 2020. PMID: 32982762 Free PMC article.
-
Muscle in children with cerebral palsy: current evidence, knowledge gaps, and emerging research opportunities : Focus on Cerebral Palsy.Pediatr Res. 2024 Aug 7. doi: 10.1038/s41390-024-03422-x. Online ahead of print. Pediatr Res. 2024. PMID: 39112788 No abstract available.
References
-
- Alexander RM. Elastic Mechanisms in Animal Movement. Cambridge, MA: Cambridge University Press, 1988.
-
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204: 1529–1536, 2001. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

