Elevated Inflammatory Plasma Biomarkers in Patients With Fabry Disease: A Critical Link to Heart Failure With Preserved Ejection Fraction
- PMID: 30571380
- PMCID: PMC6404196
- DOI: 10.1161/JAHA.118.009098
Elevated Inflammatory Plasma Biomarkers in Patients With Fabry Disease: A Critical Link to Heart Failure With Preserved Ejection Fraction
Abstract
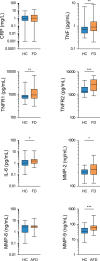
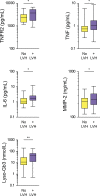
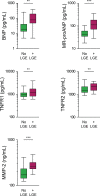
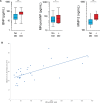
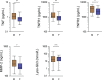
Background Because systemic inflammation and endothelial dysfunction lead to heart failure with preserved ejection fraction, we characterized plasma levels of inflammatory and cardiac remodeling biomarkers in patients with Fabry disease ( FD ). Methods and Results Plasma biomarkers were studied in multicenter cohorts of patients with FD (n=68) and healthy controls (n=40). Plasma levels of the following markers of inflammation and cardiac remodeling were determined: tumor necrosis factor ( TNF ), TNF receptor 1 ( TNFR 1) and 2 ( TNFR 2), interleukin-6, matrix metalloprotease-2 ( MMP -2), MMP -8, MMP -9, galectin-1, galectin-3, B-type natriuretic peptide ( BNP ), midregional pro-atrial natriuretic peptide ( MR -pro ANP ), and globotriaosylsphingosine. Clinical profile, cardiac magnetic resonance imaging, and echocardiogram were reviewed and correlated with biomarkers. Patients with FD had elevated plasma levels of BNP , MR -pro ANP , MMP -2, MMP -9, TNF , TNFR 1, TNFR 2, interleukin-6, galectin-1, globotriaosylsphingosine, and analogues. Plasma TNFR 2, TNF , interleukin-6, MMP -2, and globotriaosylsphingosine were elevated in FD patients with left ventricular hypertrophy, whereas diastolic dysfunction correlated with higher BNP , MR -pro ANP , and MMP -2 levels. Patients with late gadolinium enhancement on cardiac magnetic resonance imaging had greater levels of BNP , MR -pro ANP , TNFR 1, TNFR 2, and MMP -2. Plasma BNP , MR -pro ANP , MMP -2, MMP -8, TNF , TNFR 1, TNFR 2, galectin-1, and galectin-3 were elevated in patients with renal dysfunction. Patients undergoing enzyme replacement therapy who have more severe disease had higher MMP -2, TNF , TNFR 1, TNFR 2, and globotriaosylsphingosine analogue levels. Conclusions Inflammatory and cardiac remodeling biomarkers are elevated in FD patients and correlate with disease progression. These features are consistent with a phenotype dominated by heart failure with preserved ejection fraction and suggest a key pathogenic role of systemic inflammation in FD .
Keywords: Fabry disease; biomarkers; heart failure with preserved ejection fraction; hypertrophy; inflammation.
Figures




References
-
- Niemann M, Rolfs A, Stork S, Bijnens B, Breunig F, Beer M, Ertl G, Wanner C, Weidemann F. Gene mutations versus clinically relevant phenotypes: lyso‐Gb3 defines Fabry disease. Circ Cardiovasc Genet. 2014;7:8–16. - PubMed
-
- Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, Lin SJ, Chen CH, Chiang CC, Ho HJ, Lee PC, Kao CH, Cheng KH, Hsueh C, Niu DM. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–456. - PubMed
-
- Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S. Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet. 2013;58:548–552. - PubMed
-
- Gambarin FI, Disabella E, Narula J, Diegoli M, Grasso M, Serio A, Favalli V, Agozzino M, Tavazzi L, Fraser AG, Arbustini E. When should cardiologists suspect Anderson‐Fabry disease? Am J Cardiol. 2010;106:1492–1499. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

