GDF11 Decreases Pressure Overload-Induced Hypertrophy, but Can Cause Severe Cachexia and Premature Death
- PMID: 30571461
- PMCID: PMC6309347
- DOI: 10.1161/CIRCRESAHA.118.312955
GDF11 Decreases Pressure Overload-Induced Hypertrophy, but Can Cause Severe Cachexia and Premature Death
Abstract
Rationale: Possible beneficial effects of GDF11 (growth differentiation factor 11) on the normal, diseased, and aging heart have been reported, including reversing aging-induced hypertrophy. These effects have not been well validated. High levels of GDF11 have also been shown to cause cardiac and skeletal muscle wasting. These controversies could be resolved if dose-dependent effects of GDF11 were defined in normal and aged animals as well as in pressure overload-induced pathological hypertrophy.
Objective: To determine dose-dependent effects of GDF11 on normal hearts and those with pressure overload-induced cardiac hypertrophy.
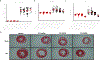
Methods and results: Twelve- to 13-week-old C57BL/6 mice underwent transverse aortic constriction (TAC) surgery. One-week post-TAC, these mice received rGDF11 (recombinant GDF11) at 1 of 3 doses: 0.5, 1.0, or 5.0 mg/kg for up to 14 days. Treatment with GDF11 increased plasma concentrations of GDF11 and p-SMAD2 in the heart. There were no significant differences in the peak pressure gradients across the aortic constriction between treatment groups at 1 week post-TAC. Two weeks of GDF11 treatment caused dose-dependent decreases in cardiac hypertrophy as measured by heart weight/tibia length ratio, myocyte cross-sectional area, and left ventricular mass. GDF11 improved cardiac pump function while preventing TAC-induced ventricular dilation and caused a dose-dependent decrease in interstitial fibrosis (in vivo), despite increasing markers of fibroblast activation and myofibroblast transdifferentiation (in vitro). Treatment with the highest dose (5.0 mg/kg) of GDF11 caused severe body weight loss, with significant decreases in both muscle and organ weights and death in both sham and TAC mice.
Conclusions: Although GDF11 treatment can reduce pathological cardiac hypertrophy and associated fibrosis while improving cardiac pump function in pressure overload, high doses of GDF11 cause severe cachexia and death. Use of GDF11 as a therapy could have potentially devastating actions on the heart and other tissues.
Keywords: cachexia; cardiomegaly; fibrosis; growth differentiation factors; hypertrophy.
Figures



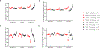


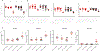
References
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. - PMC - PubMed
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK and He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. - PubMed
-
- Lakatta EG and Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 2003;107:139–46. - PubMed
-
- Lakatta EG and Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 2003;107:346–54. - PubMed
-
- Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol 1977;51:186–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

