S-Nitrosoglutathione Reductase Is Essential for Protecting the Female Heart From Ischemia-Reperfusion Injury
- PMID: 30571462
- PMCID: PMC6310045
- DOI: 10.1161/CIRCRESAHA.118.313956
S-Nitrosoglutathione Reductase Is Essential for Protecting the Female Heart From Ischemia-Reperfusion Injury
Abstract
Rationale: Protein S-nitros(yl)ation (SNO) has been implicated as an essential mediator of nitric oxide-dependent cardioprotection. Compared with males, female hearts exhibit higher baseline levels of protein SNO and associated with this, reduced susceptibility to myocardial ischemia-reperfusion injury. Female hearts also exhibit enhanced S-nitrosoglutathione reductase (GSNO-R) activity, which would typically favor decreased SNO levels as GSNO-R mediates SNO catabolism.
Objective: Because female hearts exhibit higher SNO levels, we hypothesized that GSNO-R is an essential component of sex-dependent cardioprotection in females.
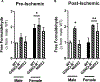
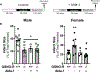
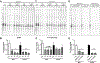
Methods and results: Male and female wild-type mouse hearts were subjected to ex vivo ischemia-reperfusion injury with or without GSNO-R inhibition (N6022). Control female hearts exhibited enhanced functional recovery and decreased infarct size versus control males. Interestingly, GSNO-R inhibition reversed this sex disparity, significantly reducing injury in male hearts, and exacerbating injury in females. Similar results were obtained with male and female GSNO-R-/- hearts using ex vivo and in vivo models of ischemia-reperfusion injury. Assessment of SNO levels using SNO-resin assisted capture revealed an increase in total SNO levels with GSNO-R inhibition in males, whereas total SNO levels remained unchanged in females. However, we found that although GSNO-R inhibition significantly increased SNO at the cardioprotective Cys39 residue of nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit 3 in males, SNO-NADH dehydrogenase subunit 3 levels were surprisingly reduced in N6022-treated female hearts. Because GSNO-R also acts as a formaldehyde dehydrogenase, we examined postischemic formaldehyde levels and found that they were nearly 2-fold higher in N6022-treated female hearts compared with nontreated hearts. Importantly, the mitochondrial aldehyde dehydrogenase 2 activator, Alda-1, rescued the phenotype in GSNO-R-/- female hearts, significantly reducing infarct size.
Conclusions: These striking findings point to GSNO-R as a critical sex-dependent mediator of myocardial protein SNO and formaldehyde levels and further suggest that different therapeutic strategies may be required to combat ischemic heart disease in males and females.
Keywords: formaldehyde; heart; nitric oxide; reactive oxygen species; reperfusion injury.
Conflict of interest statement
DISCLOSURES
The authors have no potential conflicts of interest, financial or otherwise.
Figures



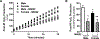

Similar articles
-
S-Nitrosoglutathione Mimics the Beneficial Activity of Endothelial Nitric Oxide Synthase-Derived Nitric Oxide in a Mouse Model of Stroke.J Stroke Cerebrovasc Dis. 2019 Dec;28(12):104470. doi: 10.1016/j.jstrokecerebrovasdis.2019.104470. Epub 2019 Nov 1. J Stroke Cerebrovasc Dis. 2019. PMID: 31680031
-
Adenosine A1 receptor activation increases myocardial protein S-nitrosothiols and elicits protection from ischemia-reperfusion injury in male and female hearts.PLoS One. 2017 May 11;12(5):e0177315. doi: 10.1371/journal.pone.0177315. eCollection 2017. PLoS One. 2017. PMID: 28493997 Free PMC article.
-
Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury.J Mol Cell Cardiol. 2007 Apr;42(4):812-25. doi: 10.1016/j.yjmcc.2007.01.010. Epub 2007 Jan 31. J Mol Cell Cardiol. 2007. PMID: 17350035 Free PMC article.
-
The role of mitochondrial reactive oxygen species, NO and H2 S in ischaemia/reperfusion injury and cardioprotection.J Cell Mol Med. 2020 Jun;24(12):6510-6522. doi: 10.1111/jcmm.15279. Epub 2020 May 8. J Cell Mol Med. 2020. PMID: 32383522 Free PMC article. Review.
-
Pre-eclampsia: the Potential of GSNO Reductase Inhibitors.Curr Hypertens Rep. 2017 Mar;19(3):20. doi: 10.1007/s11906-017-0717-2. Curr Hypertens Rep. 2017. PMID: 28271419 Free PMC article. Review.
Cited by
-
Sterile inflammation induces vasculopathy and chronic lung injury in murine sickle cell disease.Free Radic Biol Med. 2024 Mar;215:112-126. doi: 10.1016/j.freeradbiomed.2024.01.052. Epub 2024 Feb 8. Free Radic Biol Med. 2024. PMID: 38336101 Free PMC article.
-
ALKBH7 mediates necrosis via rewiring of glyoxal metabolism.Elife. 2020 Aug 14;9:e58573. doi: 10.7554/eLife.58573. Elife. 2020. PMID: 32795389 Free PMC article.
-
A knock-in mutation at cysteine 144 of TRIM72 is cardioprotective and reduces myocardial TRIM72 release.J Mol Cell Cardiol. 2019 Nov;136:95-101. doi: 10.1016/j.yjmcc.2019.09.008. Epub 2019 Sep 16. J Mol Cell Cardiol. 2019. PMID: 31536744 Free PMC article.
-
Post-Translational S-Nitrosylation of Proteins in Regulating Cardiac Oxidative Stress.Antioxidants (Basel). 2020 Oct 28;9(11):1051. doi: 10.3390/antiox9111051. Antioxidants (Basel). 2020. PMID: 33126514 Free PMC article. Review.
-
MTORC1-Regulated Metabolism Controlled by TSC2 Limits Cardiac Reperfusion Injury.Circ Res. 2021 Mar 5;128(5):639-651. doi: 10.1161/CIRCRESAHA.120.317710. Epub 2021 Jan 6. Circ Res. 2021. PMID: 33401933 Free PMC article.
References
-
- Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. - PubMed
-
- Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. - PubMed
-
- Stamler JS, Lamas S, Fang FC Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106:675–683 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

