The maternal hormone in the male brain: Sexually dimorphic distribution of prolactin signalling in the mouse brain
- PMID: 30571750
- PMCID: PMC6301622
- DOI: 10.1371/journal.pone.0208960
The maternal hormone in the male brain: Sexually dimorphic distribution of prolactin signalling in the mouse brain
Abstract
Research of the central actions of prolactin is highly focused on females, but this hormone has also documented roles in male physiology and behaviour. Here, we provide the first description of the pattern of prolactin-derived signalling in the male mouse brain, employing the immunostaining of phosphorylated signal transducer and activator of transcription 5 (pSTAT5) after exogenous prolactin administration. Next, we explore possible sexually dimorphic differences by comparing pSTAT5 immunoreactivity in prolactin-supplemented males and females. We also assess the role of testosterone in the regulation of central prolactin signalling in males by comparing intact with castrated prolactin-supplemented males. Prolactin-supplemented males displayed a widespread pattern of pSTAT5 immunoreactivity, restricted to brain centres showing expression of the prolactin receptor. Immunoreactivity for pSTAT5 was present in several nuclei of the preoptic, anterior and tuberal hypothalamus, as well as in the septofimbrial nucleus or posterodorsal medial amygdala of the telencephalon. Conversely, non-supplemented control males were virtually devoid of pSTAT5-immunoreactivity, suggesting that central prolactin actions in males are limited to situations concurrent with substantial hypophyseal prolactin release (e.g. stress or mating). Furthermore, comparison of prolactin-supplemented males and females revealed a significant, female-biased sexual dimorphism, supporting the view that prolactin has a preeminent role in female physiology and behaviour. Finally, in males, castration significantly reduced pSTAT5 immunoreactivity in some structures, including the paraventricular and ventromedial hypothalamic nuclei and the septofimbrial region, thus indicating a region-specific regulatory role of testosterone over central prolactin signalling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
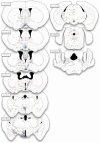

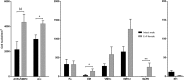
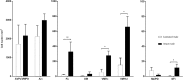
Similar articles
-
Tuning the brain for motherhood: prolactin-like central signalling in virgin, pregnant, and lactating female mice.Brain Struct Funct. 2017 Mar;222(2):895-921. doi: 10.1007/s00429-016-1254-5. Epub 2016 Jun 25. Brain Struct Funct. 2017. PMID: 27344140
-
Prolactin receptor-mediated activation of pSTAT5 in the pregnant mouse brain.J Neuroendocrinol. 2020 Nov;32(11):e12901. doi: 10.1111/jne.12901. Epub 2020 Sep 30. J Neuroendocrinol. 2020. PMID: 33000513
-
Possible crosstalk between leptin and prolactin during pregnancy.Neuroscience. 2014 Feb 14;259:71-83. doi: 10.1016/j.neuroscience.2013.11.050. Epub 2013 Dec 4. Neuroscience. 2014. PMID: 24316468
-
Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications.Brain Res Brain Res Rev. 2001 Nov;37(1-3):178-200. doi: 10.1016/s0165-0173(01)00118-7. Brain Res Brain Res Rev. 2001. PMID: 11744086 Review.
-
Testosterone metabolism in the avian hypothalamus.J Steroid Biochem Mol Biol. 1991;40(4-6):557-70. doi: 10.1016/0960-0760(91)90277-c. J Steroid Biochem Mol Biol. 1991. PMID: 1958558 Review.
Cited by
-
Becoming a mother shifts the activity of the social and motivation brain networks in mice.iScience. 2022 Jun 3;25(7):104525. doi: 10.1016/j.isci.2022.104525. eCollection 2022 Jul 15. iScience. 2022. PMID: 35754727 Free PMC article.
-
Identification of differential hypothalamic DNA methylation and gene expression associated with sexual partner preferences in rams.PLoS One. 2022 May 12;17(5):e0263319. doi: 10.1371/journal.pone.0263319. eCollection 2022. PLoS One. 2022. PMID: 35552544 Free PMC article.
-
Spinal muscular atrophy: Broad disease spectrum and sex-specific phenotypes.Biochim Biophys Acta Mol Basis Dis. 2021 Apr 1;1867(4):166063. doi: 10.1016/j.bbadis.2020.166063. Epub 2021 Jan 5. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33412266 Free PMC article. Review.
-
Comparative Transcriptomics Reveal Key Sheep (Ovis aries) Hypothalamus LncRNAs that Affect Reproduction.Animals (Basel). 2019 Apr 8;9(4):152. doi: 10.3390/ani9040152. Animals (Basel). 2019. PMID: 30965601 Free PMC article.
-
Inhibition of the medial amygdala disrupts escalated aggression in lactating female mice after repeated exposure to male intruders.Commun Biol. 2022 Sep 16;5(1):980. doi: 10.1038/s42003-022-03928-2. Commun Biol. 2022. PMID: 36114351 Free PMC article.
References
-
- Riddle O, Bates R, Dykshorn S. The preparation, identification and assay of prolactin—a hormone of anterior pituitary. Am J Physiol. 1933;(105):191–216.
-
- Bole-feysot C, Goffin V, Edery M, Binart N, Kelly P a. Prolactin (PRL) and Its Receptor: Actions, Signal PRL Receptor Knockout Mice. Endocr Rev. 1998;19(February):225–68. - PubMed
-
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev [Internet]. 2000. October [cited 2015 Sep 16];80(4):1523–631. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11015620 10.1152/physrev.2000.80.4.1523 - DOI - PubMed
-
- Salais-López H, Lanuza E, Agustín-Pavón C, Martínez-García F. Tuning the brain for motherhood: prolactin-like central signalling in virgin, pregnant, and lactating female mice. Brain Struct Funct [Internet]. 2017. March 25 [cited 2017 Aug 28];222(2):895–921. Available from: http://link.springer.com/10.1007/s00429-016-1254-5 10.1007/s00429-016-1254-5 - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

