Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain
- PMID: 30571942
- PMCID: PMC6312407
- DOI: 10.1016/j.neuron.2018.11.009
Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain
Abstract
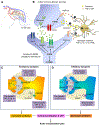
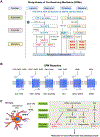
The previous decade has seen a rapid increase in microglial studies on pain, with a unique focus on microgliosis in the spinal cord after nerve injury and neuropathic pain. Numerous signaling molecules are altered in microglia and contribute to the pathogenesis of pain. Here, we discuss how microglial signaling regulates spinal cord synaptic plasticity in acute and chronic pain conditions with different degrees and variations of microgliosis. We highlight that microglial mediators such as pro- and anti-inflammatory cytokines are powerful neuromodulators that regulate synaptic transmission and pain via neuron-glial interactions. We also reveal an emerging role of microglia in the resolution of pain, in part via specialized pro-resolving mediators including resolvins, protectins, and maresins. We also discuss a possible role of microglia in chronic itch.
Keywords: central sensitization; inflammation; itch; microglia; nerve injury; neuroinflammation; neuropathic pain; specialized pro-resolving mediators; spinal cord; synaptic plasticity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

