Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback
- PMID: 30572110
- PMCID: PMC6431555
- DOI: 10.1016/j.neuroimage.2018.12.022
Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback
Abstract
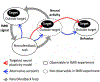
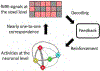
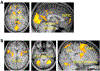
Real-time functional magnetic resonance imaging (fMRI) neurofeedback is an experimental framework in which fMRI signals are presented to participants in a real-time manner to change their behaviors. Changes in behaviors after real-time fMRI neurofeedback are postulated to be caused by neural plasticity driven by the induction of specific targeted activities at the neuronal level (targeted neural plasticity model). However, some research groups argued that behavioral changes in conventional real-time fMRI neurofeedback studies are explained by alternative accounts, including the placebo effect and physiological artifacts. Recently, decoded neurofeedback (DecNef) has been developed as a result of adapting new technological advancements, including implicit neurofeedback and fMRI multivariate analyses. DecNef provides strong evidence for the targeted neural plasticity model while refuting the abovementioned alternative accounts. In this review, we first discuss how DecNef refutes the alternative accounts. Second, we propose a model that shows how targeted neural plasticity occurs at the neuronal level during DecNef training. Finally, we discuss computational and empirical evidence that supports the model. Clarification of the neural mechanisms of DecNef would lead to the development of more advanced fMRI neurofeedback methods that may serve as powerful tools for both basic and clinical research.
Keywords: Decoded neurofeedback (DecNef); Functional magnetic resonance imaging (fMRI).
Copyright © 2018 Brain Information Communication Research Laboratory Group, Advanced Telecommunications Research Institutes International. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest
A potential financial conflict of interest exists; the authors are the inventors of patents related to the decoded neurofeedback method discussed in this article, while the original assignee of the patents is ATR, with which the authors are affiliated.
Figures



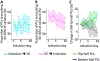





References
-
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF, 2007. Learning the value of information in an uncertain world. Nat. Neurosci 10, 1214–1221. - PubMed
-
- Behroozi M, Daliri MR, Boyaci H, 2011. Statistical analysis methods for the fMRI data. Basic Clin. Neurosci 2, 67–74.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

